Quality and quantity control at the endoplasmic reticulum
- PMID: 20570125
- PMCID: PMC2929805
- DOI: 10.1016/j.ceb.2010.05.005
Quality and quantity control at the endoplasmic reticulum
Abstract
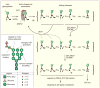
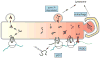
The endoplasmic reticulum (ER) is the site of maturation for secretory and membrane proteins that together make up about one third of the cellular proteome. Cells carefully control the synthetic output of this organelle to regulate both quality and quantity of proteins that emerge. Here, we synthesize current concepts underlying the pathways that mediate protein degradation from the ER and their deployment under physiologic and pathologic conditions.
Published by Elsevier Ltd.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

