Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors
- PMID: 20570248
- PMCID: PMC2907468
- DOI: 10.1016/j.biopsych.2010.04.016
Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors
Abstract
Background: Modulation of midbrain dopamine neurons by nicotinic acetylcholine receptors (nAChRs) plays an important role in behavior, cognition, motivation, and reward. Specifically, nAChRs containing beta2 subunits (beta2-nAChRs) switch dopamine cells from a resting to an excited state. However, how beta2-nAChRs can be modulated and thereby how dopamine firing activity is affected remains elusive. Because changes in dopamine cell activity are reflected in the dynamics of microcircuits generating altered responses to stimuli and inputs, factors regulating their state are fundamental. Among these, endogenous ligands to the nuclear receptor-transcription factor peroxisome proliferator-activated receptors type-alpha (PPARalpha) have been recently found to suppress nicotine-induced responses of dopamine neurons.
Methods: We used both in vitro and in vivo electrophysiological techniques together with behavioral analysis to investigate on the effects of modulation of PPARalpha in Sprague-Dawley rat and C57BLJ/6 mouse dopamine neurons and their interactions with beta2-nAChRs. To this aim, we took advantage of a selective reexpression of beta2-nAChR exclusively in dopamine cells by stereotaxically injecting a lentiviral vector in the mouse ventral tegmental area.
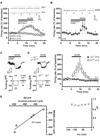
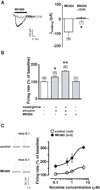
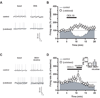
Results: We found that activation of PPARalpha decreases in vitro both dopamine cell activity and ventral tegmental area net output through negative modulation of beta2-nAChRs. Additionally, PPARalpha activation in vivo reduces both the number of spontaneously active dopamine neurons and nicotine-induced increased locomotion.
Conclusions: Our combined findings suggest PPARalpha ligands as important negative modulators of beta2-nAChRs on dopamine neurons. Thus, PPARalpha ligands might prove beneficial in treating disorders in which dopamine dysfunction plays a prominent role, such as schizophrenia and nicotine addiction.
Copyright 2010 Society of Biological Psychiatry. All rights reserved.
Conflict of interest statement
The authors reported no biomedical financial interests or potential conflicts of interest.
Figures



Similar articles
-
PPARα regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving α7 nicotinic acetylcholine receptors.J Neurosci. 2013 Apr 3;33(14):6203-11. doi: 10.1523/JNEUROSCI.4647-12.2013. J Neurosci. 2013. PMID: 23554501 Free PMC article.
-
PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors.Neuropharmacology. 2016 Nov;110(Pt A):251-259. doi: 10.1016/j.neuropharm.2016.07.024. Epub 2016 Jul 22. Neuropharmacology. 2016. PMID: 27457507
-
Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas.J Neurosci. 2003 Apr 15;23(8):3176-85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. J Neurosci. 2003. PMID: 12716925 Free PMC article.
-
Physiological role of peroxisome proliferator-activated receptors type α on dopamine systems.CNS Neurol Disord Drug Targets. 2013 Feb 1;12(1):70-7. doi: 10.2174/1871527311312010012. CNS Neurol Disord Drug Targets. 2013. PMID: 23394525 Review.
-
Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology.Acta Pharmacol Sin. 2009 Jun;30(6):740-51. doi: 10.1038/aps.2009.63. Acta Pharmacol Sin. 2009. PMID: 19498417 Free PMC article. Review.
Cited by
-
The PPARα Agonist Fenofibrate Reduces Prepulse Inhibition Disruption in a Neurodevelopmental Model of Schizophrenia.Schizophr Res Treatment. 2012;2012:839853. doi: 10.1155/2012/839853. Epub 2012 May 15. Schizophr Res Treatment. 2012. PMID: 22966448 Free PMC article.
-
PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption.Neuropharmacology. 2014 Nov;86:397-407. doi: 10.1016/j.neuropharm.2014.06.024. Epub 2014 Jul 15. Neuropharmacology. 2014. PMID: 25036611 Free PMC article.
-
Fenofibrate Administration Reduces Alcohol and Saccharin Intake in Rats: Possible Effects at Peripheral and Central Levels.Front Behav Neurosci. 2017 Jul 14;11:133. doi: 10.3389/fnbeh.2017.00133. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28769774 Free PMC article.
-
In vivo interactions between α7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-α: Implication for nicotine dependence.Neuropharmacology. 2017 May 15;118:38-45. doi: 10.1016/j.neuropharm.2017.03.005. Epub 2017 Mar 7. Neuropharmacology. 2017. PMID: 28279662 Free PMC article.
-
The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation.Cells. 2021 Mar 7;10(3):586. doi: 10.3390/cells10030586. Cells. 2021. PMID: 33799988 Free PMC article. Review.
References
-
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. - PubMed
-
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. - PubMed
-
- Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. - PubMed
-
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

