The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents
- PMID: 20570444
- PMCID: PMC2958232
- DOI: 10.1016/j.ctrv.2010.05.001
The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents
Abstract
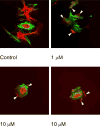
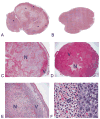
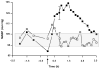
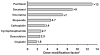
The vasculature of solid tumors is fundamentally different from that of normal vasculature and offers a unique target for anti-cancer therapy. Direct vascular-targeting with Tumor-Vascular Disrupting Agents (Tumor-VDAs) is distinctly different from anti-angiogenic strategies, and offers a complementary approach to standard therapies. Tumor-VDAs therefore have significant potential when combined with chemotherapy, radiotherapy, and angiogenesis-inhibiting agents. Preclinical studies with the different Tumor-VDA classes have demonstrated key tumor-selective anti-vascular and anti-tumor effects.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The author is a member of the Scientific Advisory Board of OXiGENE, Inc.
Figures









References
-
- McDonald D, Choyke P. Imaging of angiogenesis: from microscope to clinic. Nature Med. 2003;9:713–725. - PubMed
-
- Leu A, Berk D, Lymboussaki A, et al. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. - PubMed
-
- Padera T, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

