Glutamine addiction: a new therapeutic target in cancer
- PMID: 20570523
- PMCID: PMC2917518
- DOI: 10.1016/j.tibs.2010.05.003
Glutamine addiction: a new therapeutic target in cancer
Abstract
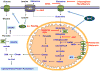
Most cancers depend on a high rate of aerobic glycolysis for their continued growth and survival. Paradoxically, some cancer cell lines also display addiction to glutamine despite the fact that glutamine is a nonessential amino acid that can be synthesized from glucose. The high rate of glutamine uptake exhibited by glutamine-dependent cells does not appear to result solely from its role as a nitrogen donor in nucleotide and amino acid biosynthesis. Instead, glutamine plays a required role in the uptake of essential amino acids and in maintaining activation of TOR (target of rapamycin) kinase. Moreover, in many cancer cells, glutamine is the primary mitochondrial substrate and is required for maintenance of mitochondrial membrane potential and integrity and for support of the NADPH production needed for redox control and macromolecular synthesis.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

