Synthesis and structure-affinity relationships of novel small molecule natural product derivatives capable of discriminating between serotonin 5-HT1A, 5-HT2A, 5-HT2C receptor subtypes
- PMID: 20570529
- PMCID: PMC2946983
- DOI: 10.1016/j.bmc.2010.05.017
Synthesis and structure-affinity relationships of novel small molecule natural product derivatives capable of discriminating between serotonin 5-HT1A, 5-HT2A, 5-HT2C receptor subtypes
Abstract
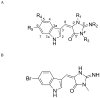
Efforts to develop ligands that distinguish between clinically relevant 5-HT2A and 5-HT2C serotonin receptor subtypes have been challenging, because their sequences have high homology. Previous studies reported that a novel aplysinopsin belonging to a chemical class of natural products isolated from a marine sponge was selective for the 5-HT2C over the 5-HT2A receptor subtype. Our goal was to explore the 5-HT2A/2C receptor structure-affinity relationships of derivatives based on the aplysinopsin natural product pharmacophore. Twenty aplysinopsin derivatives were synthesized, purified and tested for their affinities for cloned human serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptor subtypes. Four compounds in this series had >30-fold selectivity for 5-HT2A or 5-HT2C receptors. The compound (E)-5-((5,6-dichloro-1H-indol-3-yl)methylene)-2-imino-1,3-dimethylimidazolidin-4-one (UNT-TWU-22, 16) had approximately 2100-fold selectivity for the serotonin 5-HT2C receptor subtype: an affinity for 5-HT2C equal to 46 nM and no detectable affinity for the 5-HT1A or 5-HT2A receptor subtypes. The two most important factors controlling 5-HT2A or 5-HT2C receptor subtype selectivity were the combined R1,R3-alkylation of the imidazolidinone ring and the type and number of halogens on the indole ring of the aplysinopsin pharmacophore.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Kazlauskas R, Murphy PT, Quinn RJ, Wells RJ. Tetrahedron Lett. 1977;18:61–64.
-
- Hollenbeak KH, Schmitz FJ. Lloydia. 1977;40:479–481. - PubMed
-
- Djura P, Faulkner DJ. J Org Chem. 1980;45:735–737.
-
- Aoki S, Ye Y, Higuchi K, Takashima A, Tanaka Y, Kitagawa I, Kobayashi M. Chem Pharm Bull (Tokyo) 2001;49:1372–1374. - PubMed
-
- Hu JF, Schetz JA, Kelly M, Peng JN, Ang KK, Flotow H, Leong CY, Ng SB, Buss AD, Wilkins SP, Hamann MT. J Nat Prod. 2002;65:476–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

