1-Benzyl-indole-3-carbinol is a novel indole-3-carbinol derivative with significantly enhanced potency of anti-proliferative and anti-estrogenic properties in human breast cancer cells
- PMID: 20570586
- PMCID: PMC3422669
- DOI: 10.1016/j.cbi.2010.05.015
1-Benzyl-indole-3-carbinol is a novel indole-3-carbinol derivative with significantly enhanced potency of anti-proliferative and anti-estrogenic properties in human breast cancer cells
Abstract
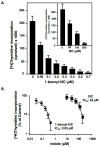
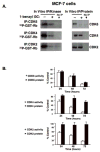
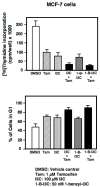
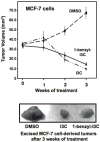
Indole-3-carbinol (I3C), a natural autolysis product of a gluccosinolate present in Brassica vegetables such as broccoli and cabbage, has anti-proliferative and anti-estrogenic activities in human breast cancer cells. A new and significantly more potent I3C analogue, 1-benzyl-I3C was synthesized, and in comparison to I3C, this novel derivative displayed an approximate 1000-fold enhanced potency in suppressing the growth of both estrogen responsive (MCF-7) and estrogen-independent (MDA-MB-231) human breast cancer cells (I3C IC(50) of 52 microM, and 1-benzyl-I3C IC(50) of 0.05 microM). At significantly lower concentrations, 1-benzyl-I3C induced a robust G1 cell cycle arrest and elicited the key I3C-specific effects on expression and activity of G1-acting cell cycle genes including the disruption of endogenous interactions of the Sp1 transcription factor with the CDK6 promoter. Furthermore, in estrogen responsive MCF-7 cells, with enhanced potency 1-benzyl-I3C down-regulated production of estrogen receptor-alpha protein, acts with tamoxifen to arrest breast cancer cell growth more effectively than either compound alone, and inhibited the in vivo growth of human breast cancer cell-derived tumor xenografts in athymic mice. Our results implicate 1-benzyl-I3C as a novel, potent inhibitor of human breast cancer proliferation and estrogen responsiveness that could potentially be developed into a promising therapeutic agent for the treatment of indole-sensitive cancers.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the G1 cell cycle arrest of human breast cancer cells.Carcinogenesis. 2011 Sep;32(9):1315-23. doi: 10.1093/carcin/bgr116. Epub 2011 Jun 21. Carcinogenesis. 2011. PMID: 21693539 Free PMC article.
-
Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling.J Biol Chem. 1998 Feb 13;273(7):3838-47. doi: 10.1074/jbc.273.7.3838. J Biol Chem. 1998. PMID: 9461564
-
A new indole-3-carbinol tetrameric derivative inhibits cyclin-dependent kinase 6 expression, and induces G1 cell cycle arrest in both estrogen-dependent and estrogen-independent breast cancer cell lines.Cancer Res. 2003 Jul 15;63(14):4028-36. Cancer Res. 2003. PMID: 12874002
-
Indole-3-carbinol and 3-3'-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions.J Nutr. 2003 Jul;133(7 Suppl):2448S-2455S. doi: 10.1093/jn/133.7.2448S. J Nutr. 2003. PMID: 12840223 Review.
-
Molecular targets and anticancer potential of indole-3-carbinol and its derivatives.Cell Cycle. 2005 Sep;4(9):1201-15. doi: 10.4161/cc.4.9.1993. Epub 2005 Sep 6. Cell Cycle. 2005. PMID: 16082211 Review.
Cited by
-
Essential role of the cancer stem/progenitor cell marker nucleostemin for indole-3-carbinol anti-proliferative responsiveness in human breast cancer cells.BMC Biol. 2014 Sep 12;12:72. doi: 10.1186/s12915-014-0072-6. BMC Biol. 2014. PMID: 25209720 Free PMC article.
-
Artemisinin triggers a G1 cell cycle arrest of human Ishikawa endometrial cancer cells and inhibits cyclin-dependent kinase-4 promoter activity and expression by disrupting nuclear factor-κB transcriptional signaling.Anticancer Drugs. 2014 Mar;25(3):270-81. doi: 10.1097/CAD.0000000000000054. Anticancer Drugs. 2014. PMID: 24296733 Free PMC article.
-
Simultaneous inhibition of cell-cycle, proliferation, survival, metastatic pathways and induction of apoptosis in breast cancer cells by a phytochemical super-cocktail: genes that underpin its mode of action.J Cancer. 2013 Nov 14;4(9):703-15. doi: 10.7150/jca.7235. eCollection 2013. J Cancer. 2013. PMID: 24312140 Free PMC article.
-
1-Benzyl-indole-3-carbinol is a highly potent new small molecule inhibitor of Wnt/β-catenin signaling in melanoma cells that coordinately inhibits cell proliferation and disrupts expression of microphthalmia-associated transcription factor isoform-M.Carcinogenesis. 2017 Dec 7;38(12):1207-1217. doi: 10.1093/carcin/bgx103. Carcinogenesis. 2017. PMID: 29028954 Free PMC article.
-
The indole-3-carbinol cyclic tetrameric derivative CTet inhibits cell proliferation via overexpression of p21/CDKN1A in both estrogen receptor-positive and triple-negative breast cancer cell lines.Breast Cancer Res. 2011 Mar 24;13(2):R33. doi: 10.1186/bcr2855. Breast Cancer Res. 2011. PMID: 21435243 Free PMC article.
References
-
- van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol. 1999;472:159–168. - PubMed
-
- Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA. 2001;285:2975–2977. - PubMed
-
- Lopez-Otin C, Diamandis EP. Breast and prostate cancer: an analysis of common epidemiological, genetic, and biochemical features. Endocr Rev. 1998;19:365–396. - PubMed
-
- Moiseeva EP, Manson MM. Dietary chemopreventive phytochemicals: too little or too much? Cancer Prev Res. 2009;2:611–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

