Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress
- PMID: 20570728
- PMCID: PMC4712644
- DOI: 10.1016/j.cellsig.2010.05.024
Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress
Abstract
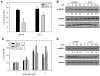
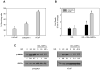
Androgen-independent prostate cancer usually develops as a relapse following androgen ablation therapy. Removing androgen systemically causes vascular degeneration and nutrient depletion of the prostate tumor tissue. The fact that the malignancy later evolves to androgen-independence suggests that some cancer cells are able to survive the challenge of energy/nutrient deprivation. AMP-activated protein kinase (AMPK) is an important manager of energy stress. The present study was designed to investigate the role of AMPK in contributing to the survival of the androgen-independent phenotype. Most of the experiments were carried out in the androgen-dependent LNCaP cells and the androgen-independent C4-2 cells. These two cell lines have the same genetic background, since the C4-2 line is derived from the LNCaP line. Glucose deprivation (GD) was instituted to model energy stress encountered by these cells. The key findings are as follows. First, the activation of AMPK by GD was much stronger in C4-2 cells than in LNCaP cells, and the robustness of AMPK activation was correlated favorably with cell viability. Second, the response of AMPK was specific to energy deficiency rather than to amino acid deficiency. The activation of AMPK by GD was functional, as demonstrated by appropriate phosphorylation changes of mTOR and mTOR downstream substrates. Third, blocking AMPK activation by chemical inhibitor or dominant negative AMPK led to increased apoptotic cell death. The observation that similar results were found in other androgen-independent prostate cancer cell lines, including CW22Rv1 abd VCaP, provided further assurance that AMPK is a facilitator on the road to androgen-independence of prostate cancer cells.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

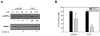



References
-
- Helmlinger G, Yuan F, Dellian M, Jain RK. Nat Med. 1997;3:177. - PubMed
-
- Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Cancer Res. 2000;60:6201. - PubMed
-
- Hardie DG. Nat Rev Mol Cell Biol. 2007;8:774. - PubMed
-
- McGee SL, Hargreaves M. Front Biosci. 2008;13:3022. - PubMed
-
- Hardie DG, Scott JW, Pan DA, Hudson ER. FEBS Lett. 2003;546:113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

