Genetic, hormonal, and metabolomic influences on social behavior and sex preference of XXY mice
- PMID: 20570823
- PMCID: PMC2944286
- DOI: 10.1152/ajpendo.00085.2010
Genetic, hormonal, and metabolomic influences on social behavior and sex preference of XXY mice
Abstract
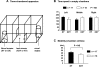
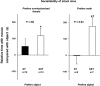
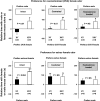
XXY men (Klinefelter syndrome) are testosterone deficient, socially isolated, exhibit impaired gender identity, and may experience more homosexual behaviors. Here, we characterize social behaviors in a validated XXY mouse model to understand mechanisms. Sociability and gender preference were assessed by three-chambered choice tasks before and after castration and after testosterone replacement. Metabolomic activities of brain and blood were quantified through fractional synthesis rates of palmitate and ribose (GC-MS). XXY mice exhibit greater sociability than XY littermates, particularly for male mice. The differences in sociability disappear after matching androgen exposure. Intact XXY, compared with XY, mice prefer male mice odors when the alternatives are ovariectomized female mice odors, but they prefer estrous over male mice odors, suggesting that preference for male mice may be due to social, not sexual, cues. Castration followed by testosterone treatment essentially remove these preferences. Fractional synthesis rates of palmitate are higher in the hypothalamus, amygdala, and hippocampus of XXY compared with XY mice but not with ribose in these brain regions or palmitate in blood. Androgen ablation in XY mice increases fractional synthesis rates of fatty acids in the brain to levels indistinguishable from those in XXY mice. We conclude that intact XXY mice exhibit increased sociability, differences in gender preference for mice and their odors are due to social rather than sexual cues and, these differences are mostly related to androgen deficiency rather than genetics. Specific metabolic changes in brain lipids, which are also regulated by androgens, are observed in brain regions that are involved in these behaviors.
Figures


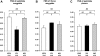
Similar articles
-
Genetic and hormonal control of bone volume, architecture, and remodeling in XXY mice.J Bone Miner Res. 2010 Oct;25(10):2148-54. doi: 10.1002/jbmr.104. J Bone Miner Res. 2010. PMID: 20499350 Free PMC article.
-
Non-neural androgen receptor promotes androphilic odor preference in mice.Horm Behav. 2016 Jul;83:14-22. doi: 10.1016/j.yhbeh.2016.05.013. Epub 2016 May 15. Horm Behav. 2016. PMID: 27191855
-
XXY mice exhibit gonadal and behavioral phenotypes similar to Klinefelter syndrome.Endocrinology. 2005 Sep;146(9):4148-54. doi: 10.1210/en.2005-0278. Epub 2005 Jun 16. Endocrinology. 2005. PMID: 15961558
-
Insights into the pathogenesis of XXY phenotype from comparison of the clinical syndrome with an experimental XXY mouse model.Pediatr Endocrinol Rev. 2010 Dec;8 Suppl 1:140-4. Pediatr Endocrinol Rev. 2010. PMID: 21217605 Review.
-
Odor-guided behavior in mammals.Experientia. 1986 Mar 15;42(3):257-71. doi: 10.1007/BF01942506. Experientia. 1986. PMID: 3514263 Review.
Cited by
-
Number of X-chromosome genes influences social behavior and vasopressin gene expression in mice.Psychoneuroendocrinology. 2015 Jan;51:271-81. doi: 10.1016/j.psyneuen.2014.10.010. Epub 2014 Oct 23. Psychoneuroendocrinology. 2015. PMID: 25462900 Free PMC article.
-
Feminized behavior and brain gene expression in a novel mouse model of Klinefelter Syndrome.Arch Sex Behav. 2014 Aug;43(6):1043-57. doi: 10.1007/s10508-014-0316-0. Epub 2014 Jun 13. Arch Sex Behav. 2014. PMID: 24923877 Free PMC article.
-
Neuroanatomical and molecular correlates of cognitive and behavioural outcomes in hypogonadal males.Metab Brain Dis. 2018 Apr;33(2):491-505. doi: 10.1007/s11011-017-0163-5. Epub 2017 Dec 11. Metab Brain Dis. 2018. PMID: 29230619 Review.
-
Triangulating the sexually dimorphic brain through high-resolution neuroimaging of murine sex chromosome aneuploidies.Brain Struct Funct. 2015 Nov;220(6):3581-93. doi: 10.1007/s00429-014-0875-9. Epub 2014 Aug 22. Brain Struct Funct. 2015. PMID: 25146308 Free PMC article.
-
The Sex Chromosome Trisomy mouse model of XXY and XYY: metabolism and motor performance.Biol Sex Differ. 2013 Aug 8;4(1):15. doi: 10.1186/2042-6410-4-15. Biol Sex Differ. 2013. PMID: 23926958 Free PMC article.
References
-
- Bancroft J, Axworthy D, Ratcliffe S. The personality and psycho-sexual development of boys with 47 XXY chromosome constitution. J Child Psychol Psychiatry 23: 169–180, 1982 - PubMed
-
- Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 88: 622–626, 2003 - PubMed
-
- Boone KB, Swerdloff RS, Miller BL, Geschwind DH, Razani J, Lee A, Gonzalo IG, Haddal A, Rankin K, Lu P, Paul L. Neuropsychological profiles of adults with Klinefelter syndrome. J Int Neuropsychol Soc 7: 446–456, 2001 - PubMed
-
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature 444: 308–315, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

