Mitogen-activated protein/extracellular signal-regulated kinase kinase 1act/tubulin interaction is an important determinant of mitotic stability in cultured HT1080 human fibrosarcoma cells
- PMID: 20570892
- PMCID: PMC2938962
- DOI: 10.1158/0008-5472.CAN-09-4490
Mitogen-activated protein/extracellular signal-regulated kinase kinase 1act/tubulin interaction is an important determinant of mitotic stability in cultured HT1080 human fibrosarcoma cells
Abstract
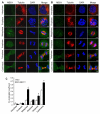
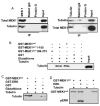
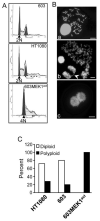
Activation of the mitogen-activated protein kinase (MAPK) pathway plays a major role in neoplastic cell transformation. Using a proteomics approach, we identified alpha tubulin and beta tubulin as proteins that interact with activated MAP/extracellular signal-regulated kinase kinase 1 (MEK1), a central MAPK regulatory kinase. Confocal analysis revealed spatiotemporal control of MEK1-tubulin colocalization that was most prominent in the mitotic spindle apparatus in variant HT1080 human fibrosarcoma cells. Peptide arrays identified the critical role of positively charged amino acids R108, R113, R160, and K157 on the surface of MEK1 for tubulin interaction. Overexpression of activated MEK1 caused defects in spindle arrangement, chromosome segregation, and ploidy. In contrast, chromosome polyploidy was reduced in the presence of an activated MEK1 mutant (R108A, R113A) that disrupted interactions with tubulin. Our findings indicate the importance of signaling by activated MEK1-tubulin in spindle organization and chromosomal instability.
(c)2010 AACR.
Figures



Similar articles
-
RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity.J Biol Chem. 2010 Aug 20;285(34):26461-74. doi: 10.1074/jbc.M110.121491. Epub 2010 Jun 17. J Biol Chem. 2010. PMID: 20558733 Free PMC article.
-
Sef downregulation by Ras causes MEK1/2 to become aberrantly nuclear localized leading to polyploidy and neoplastic transformation.Cancer Res. 2012 Feb 1;72(3):626-35. doi: 10.1158/0008-5472.CAN-11-2126. Cancer Res. 2012. PMID: 22298595
-
MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation.Cell Cycle. 2007 Feb 1;6(3):330-8. doi: 10.4161/cc.6.3.3805. Epub 2007 Feb 2. Cell Cycle. 2007. PMID: 17297311
-
Regulation of intracellular MEK1/2 translocation in mouse oocytes: cytoplasmic dynein/dynactin-mediated poleward transport and cyclin B degradation-dependent release from spindle poles.Cell Cycle. 2007 Jun 15;6(12):1521-7. doi: 10.4161/cc.6.12.4355. Epub 2007 Apr 26. Cell Cycle. 2007. PMID: 17507801
-
Intrinsic and acquired resistance to MEK1/2 inhibitors in cancer.Biochem Soc Trans. 2014 Aug;42(4):776-83. doi: 10.1042/BST20140129. Biochem Soc Trans. 2014. PMID: 25109957 Review.
Cited by
-
NAD(P)H:quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in human cells.PLoS One. 2012;7(9):e44861. doi: 10.1371/journal.pone.0044861. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984577 Free PMC article.
-
Kinome profiling.Scientifica (Cairo). 2012;2012:306798. doi: 10.6064/2012/306798. Epub 2012 Aug 7. Scientifica (Cairo). 2012. PMID: 24278683 Free PMC article. Review.
-
Divergent Polypharmacology-Driven Cellular Activity of Structurally Similar Multi-Kinase Inhibitors through Cumulative Effects on Individual Targets.Cell Chem Biol. 2019 Sep 19;26(9):1240-1252.e11. doi: 10.1016/j.chembiol.2019.06.003. Epub 2019 Jun 27. Cell Chem Biol. 2019. PMID: 31257184 Free PMC article.
-
Targeting Adenylate Cyclase Family: New Concept of Targeted Cancer Therapy.Front Oncol. 2022 Jun 27;12:829212. doi: 10.3389/fonc.2022.829212. eCollection 2022. Front Oncol. 2022. PMID: 35832555 Free PMC article. Review.
-
Spatiotemporal control of pathway sensors and cross-pathway feedback regulate a differentiation MAPK pathway in yeast.J Cell Sci. 2021 Aug 1;134(15):jcs258341. doi: 10.1242/jcs.258341. Epub 2021 Aug 4. J Cell Sci. 2021. PMID: 34347092 Free PMC article.
References
-
- Giroux S, Tremblay M, Bernard D, et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr Biol. 1999;9:369–72. - PubMed
-
- Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–26. - PubMed
-
- Scholl FA, Dumesic PA, Khavari PA. Mek1 alters epidermal growth and differentiation. Cancer Res. 2004;64:6035–40. - PubMed
-
- Scholl FA, Dumesic PA, Khavari PA. Effects of active MEK1 expression in vivo. Cancer Lett. 2005;230:1–5. - PubMed
-
- Mansour SJ, Matten WT, Hermann AS, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

