The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline
- PMID: 20570941
- PMCID: PMC2889607
- DOI: 10.1242/dev.047993
The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline
Abstract
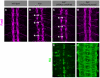
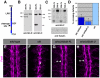
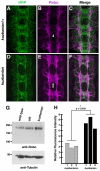
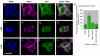
Slits and their Roundabout (Robo) receptors mediate repulsive axon guidance at the Drosophila ventral midline and in the vertebrate spinal cord. Slit is cleaved to produce fragments with distinct signaling properties. In a screen for genes involved in Slit-Robo repulsion, we have identified the Adam family metalloprotease Kuzbanian (Kuz). Kuz does not regulate midline repulsion through cleavage of Slit, nor is Slit cleavage essential for repulsion. Instead, Kuz acts in neurons to regulate repulsion and Kuz can cleave the Robo extracellular domain in Drosophila cells. Genetic rescue experiments using an uncleavable form of Robo show that this receptor does not maintain normal repellent activity. Finally, Kuz activity is required for Robo to recruit its downstream signaling partner, Son of sevenless (Sos). These observations support the model that Kuz-directed cleavage is important for Robo receptor activation.
Figures




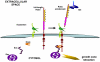
References
-
- Bashaw G. J., Goodman C. S. (1999). Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell 97, 917-926 - PubMed
-
- Battye R., Stevens A., Jacobs J. R. (1999). Axon repulsion from the midline of the Drosophila CNS requires slit function. Development 126, 2475-2481 - PubMed
-
- Becherer J. D., Blobel C. P. (2003). Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs). Curr. Top. Dev. Biol. 54, 101-123 - PubMed
-
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

