FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis
- PMID: 20570963
- PMCID: PMC4338542
- DOI: 10.1096/fj.10-159913
FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis
Abstract
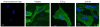
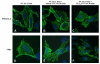
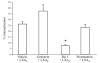
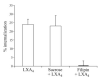
Lipoxins (LXs) are endogenously produced eicosanoids with well-described anti-inflammatory and proresolution activities, stimulating nonphlogistic phagocytosis of apoptotic cells by macrophages. LXA(4) and the glucocorticoid-derived annexin A1 peptide (Ac2-26) bind to a common G-protein-coupled receptor, termed FPR2/ALX. However, direct evidence of the involvement of FPR2/ALX in the anti-inflammatory and proresolution activity of LXA(4) is still to be investigated. Here we describe FPR2/ALX trafficking in response to LXA(4) and Ac2-26 stimulation. We have transfected cells with HA-tagged FPR2/ALX and studied receptor trafficking in unstimulated, LXA(4) (1-10 nM)- and Ac2-26 (30 μM)-treated cells using multiple approaches that include immunofluorescent confocal microscopy, immunogold labeling of cryosections, and ELISA and investigated receptor trafficking in agonist-stimulated phagocytosis. We conclude that PKC-dependent internalization of FPR2/ALX is required for phagocytosis. Using bone marrow-derived macrophages (BMDMs) from mice in which the FPR2/ALX ortholog Fpr2 had been deleted, we observed the nonredundant function for this receptor in LXA(4) and Ac2-26 stimulated phagocytosis of apoptotic neutrophils. LXA(4) stimulated phagocytosis 1.7-fold above basal (P<0.001) by BMDMs from wild-type mice, whereas no effect was found on BMDMs from Fpr2(-/-) mice. Similarly, Ac2-26 stimulates phagocytosis by BMDMs from wild-type mice 1.5-fold above basal (P<0.05). However, Ac2-26 failed to stimulate phagocytosis by BMDMs isolated from Fpr2(-/-) mice relative to vehicle. These data reveal novel and complex mechanisms of the FPR2/ALX receptor trafficking and functionality in the resolution of inflammation.
Figures

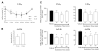
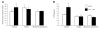
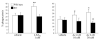
References
-
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostag. Leukot. Essent. Fatty Acids. 2005;73:141–162. - PubMed
-
- Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim. Biophys. Acta. 2003;1639:141–151. - PubMed
-
- Kieran NE, Maderna P, Godson C. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–1154. - PubMed
-
- McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am. J. Physiol. Renal Physiol. 2004;286:F189–F201. - PubMed
-
- Maderna P, Godson C. Taking insult from injury: lipoxins and lipoxin receptor agonists and phagocytosis of apoptotic cells. Prostag. Leukot. Essent. Fatty Acids. 2005;73:179–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

