Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells
- PMID: 20571029
- PMCID: PMC2924093
- DOI: 10.1074/jbc.M109.063255
Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells
Abstract
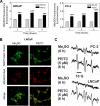
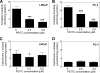
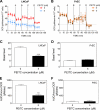
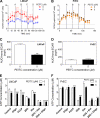
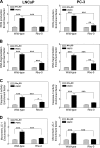
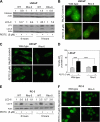
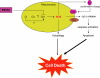
Phenethyl isothiocyanate (PEITC), a constituent of edible cruciferous vegetables such as watercress, not only affords significant protection against chemically induced cancer in experimental rodents but also inhibits growth of human cancer cells by causing apoptotic and autophagic cell death. However, the underlying mechanism of PEITC-induced cell death is not fully understood. Using LNCaP and PC-3 human prostate cancer cells as a model, we demonstrate that the PEITC-induced cell death is initiated by production of reactive oxygen species (ROS) resulting from inhibition of oxidative phosphorylation (OXPHOS). Exposure of LNCaP and PC-3 cells to pharmacologic concentrations of PEITC resulted in ROS production, which correlated with inhibition of complex III activity, suppression of OXPHOS, and ATP depletion. These effects were not observed in a representative normal human prostate epithelial cell line (PrEC). The ROS production by PEITC treatment was not influenced by cyclosporin A. The Rho-0 variants of LNCaP and PC-3 cells were more resistant to PEITC-mediated ROS generation, apoptotic DNA fragmentation, and collapse of mitochondrial membrane potential compared with respective wild-type cells. The PEITC treatment resulted in activation of Bax in wild-type LNCaP and PC-3 cells, but not in their respective Rho-0 variants. Furthermore, RNA interference of Bax and Bak conferred significant protection against PEITC-induced apoptosis. The Rho-0 variants of LNCaP and PC-3 cells also resisted PEITC-mediated autophagy. In conclusion, the present study provides novel insight into the molecular circuitry of PEITC-induced cell death involving ROS production due to inhibition of complex III and OXPHOS.
Figures




Similar articles
-
Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells.Cancer Res. 2009 Apr 15;69(8):3704-12. doi: 10.1158/0008-5472.CAN-08-4344. Epub 2009 Mar 31. Cancer Res. 2009. PMID: 19336571 Free PMC article.
-
p66Shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells.Cancer Res. 2010 Apr 15;70(8):3150-8. doi: 10.1158/0008-5472.CAN-09-4451. Epub 2010 Mar 30. Cancer Res. 2010. PMID: 20354186 Free PMC article.
-
Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential.Carcinogenesis. 2006 Nov;27(11):2223-34. doi: 10.1093/carcin/bgl087. Epub 2006 Jun 13. Carcinogenesis. 2006. PMID: 16774948
-
Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms.Biochim Biophys Acta. 2014 Dec;1846(2):405-24. doi: 10.1016/j.bbcan.2014.08.003. Epub 2014 Aug 23. Biochim Biophys Acta. 2014. PMID: 25152445 Free PMC article. Review.
-
Pharmacokinetics and pharmacodynamics of phenethyl isothiocyanate: implications in breast cancer prevention.AAPS J. 2014 Jul;16(4):705-13. doi: 10.1208/s12248-014-9610-y. Epub 2014 May 13. AAPS J. 2014. PMID: 24821055 Free PMC article. Review.
Cited by
-
Effects of Brassicaceae Isothiocyanates on Prostate Cancer.Molecules. 2016 May 12;21(5):626. doi: 10.3390/molecules21050626. Molecules. 2016. PMID: 27187332 Free PMC article. Review.
-
Potential mechanism of ferroptosis in pancreatic cancer.Oncol Lett. 2020 Jan;19(1):579-587. doi: 10.3892/ol.2019.11159. Epub 2019 Nov 28. Oncol Lett. 2020. PMID: 31897173 Free PMC article. Review.
-
Phenethyl isothiocyanate inhibits proliferation and induces apoptosis in pancreatic cancer cells in vitro and in a MIAPaca2 xenograft animal model.Nutr Cancer. 2014;66(4):747-55. doi: 10.1080/01635581.2013.795979. Epub 2013 Nov 6. Nutr Cancer. 2014. PMID: 24195616 Free PMC article.
-
Phenethyl isothiocyanate induces calcium mobilization and mitochondrial cell death pathway in cholangiocarcinoma KKU-M214 cells.BMC Cancer. 2013 Dec 5;13:571. doi: 10.1186/1471-2407-13-571. BMC Cancer. 2013. PMID: 24304591 Free PMC article.
-
Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells.Prostate. 2014 Sep;74(12):1209-21. doi: 10.1002/pros.22837. Epub 2014 Jul 7. Prostate. 2014. PMID: 25043291 Free PMC article.
References
-
- Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M. J. (2007) CA Cancer J. Clin. 57, 43–66 - PubMed
-
- Verhoeven D. T., Goldbohm R. A., van Poppel G., Verhagen H., van den Brandt P. A. (1996) Cancer Epidemiol. Biomarkers Prev. 5, 733–748 - PubMed
-
- Kolonel L. N., Hankin J. H., Whittemore A. S., Wu A. H., Gallagher R. P., Wilkens L. R., John E. M., Howe G. R., Dreon D. M., West D. W., Paffenbarger R. S., Jr. (2000) Cancer Epidemiol. Biomarkers Prev. 9, 795–804 - PubMed
-
- Ambrosone C. B., McCann S. E., Freudenheim J. L., Marshall J. R., Zhang Y., Shields P. G. (2004) J. Nutr. 134, 1134–1138 - PubMed
-
- Hecht S. S. (2000) Drug Metab. Rev. 32, 395–411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

