A flexible and efficient template format for circular consensus sequencing and SNP detection
- PMID: 20571086
- PMCID: PMC2926623
- DOI: 10.1093/nar/gkq543
A flexible and efficient template format for circular consensus sequencing and SNP detection
Abstract
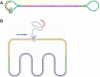
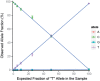
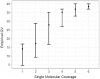
A novel template design for single-molecule sequencing is introduced, a structure we refer to as a SMRTbell template. This structure consists of a double-stranded portion, containing the insert of interest, and a single-stranded hairpin loop on either end, which provides a site for primer binding. Structurally, this format resembles a linear double-stranded molecule, and yet it is topologically circular. When placed into a single-molecule sequencing reaction, the SMRTbell template format enables a consensus sequence to be obtained from multiple passes on a single molecule. Furthermore, this consensus sequence is obtained from both the sense and antisense strands of the insert region. In this article, we present a universal method for constructing these templates, as well as an application of their use. We demonstrate the generation of high-quality consensus accuracy from single molecules, as well as the use of SMRTbell templates in the identification of rare sequence variants.
Figures

References
-
- Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138. - PubMed
-
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 2005;352:1436–1444. - PubMed
-
- Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 2009;59(Suppl. 1):S4–S16. - PubMed
-
- Orscheln RC, Hunstad DA, Fritz SA, Loughman JA, Mitchell K, Storch EK, Gaudreault M, Sellenriek PL, Armstrong JR, Mardis ER, et al. Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin. Infect. Dis. 2009;49:536–542. - PMC - PubMed
-
- Stephens AJ, Huygens F, Inman-Bamber J, Price EP, Nimmo GR, Schooneveldt J, Munckhof W, Giffard PM. Methicillin-resistant Staphylococcus aureus genotyping using a small set of polymorphisms. J. Med. Microbiol. 2006;55:43–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

