Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA
- PMID: 20571511
- PMCID: PMC2928695
- DOI: 10.1038/emboj.2010.128
Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA
Abstract
Replication-factor C (RFC) is a protein complex that loads the processivity clamp PCNA onto DNA. Elg1 is a conserved protein with homology to the largest subunit of RFC, but its function remained enigmatic. Here, we show that yeast Elg1 interacts physically and genetically with PCNA, in a manner that depends on PCNA modification, and exhibits preferential affinity for SUMOylated PCNA. This interaction is mediated by three small ubiquitin-like modifier (SUMO)-interacting motifs and a PCNA-interacting protein box close to the N-terminus of Elg1. These motifs are important for the ability of Elg1 to maintain genomic stability. SUMOylated PCNA is known to recruit the helicase Srs2, and in the absence of Elg1, Srs2 and SUMOylated PCNA accumulate on chromatin. Strains carrying mutations in both ELG1 and SRS2 exhibit a synthetic fitness defect that depends on PCNA modification. Our results underscore the importance of Elg1, Srs2 and SUMOylated PCNA in the maintenance of genomic stability.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


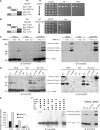
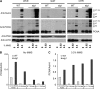


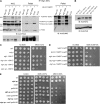
Similar articles
-
Elg1, the major subunit of an alternative RFC complex, interacts with SUMO-processing proteins.Cell Cycle. 2011 Sep 1;10(17):2894-903. doi: 10.4161/cc.10.17.16778. Epub 2011 Sep 1. Cell Cycle. 2011. PMID: 21869594
-
Access to PCNA by Srs2 and Elg1 Controls the Choice between Alternative Repair Pathways in Saccharomyces cerevisiae.mBio. 2020 May 5;11(3):e00705-20. doi: 10.1128/mBio.00705-20. mBio. 2020. PMID: 32371600 Free PMC article.
-
A structure-function analysis of the yeast Elg1 protein reveals the importance of PCNA unloading in genome stability maintenance.Nucleic Acids Res. 2017 Apr 7;45(6):3189-3203. doi: 10.1093/nar/gkw1348. Nucleic Acids Res. 2017. PMID: 28108661 Free PMC article.
-
PCNASUMO and Srs2: a model SUMO substrate-effector pair.Biochem Soc Trans. 2007 Dec;35(Pt 6):1385-8. doi: 10.1042/BST0351385. Biochem Soc Trans. 2007. PMID: 18031227 Review.
-
How yeast cells deal with stalled replication forks.Curr Genet. 2020 Oct;66(5):911-915. doi: 10.1007/s00294-020-01082-y. Epub 2020 May 11. Curr Genet. 2020. PMID: 32394094 Review.
Cited by
-
Homologous recombination maintenance of genome integrity during DNA damage tolerance.Mol Cell Oncol. 2014 Oct 29;1(2):e957039. doi: 10.4161/23723548.2014.957039. eCollection 2014 Apr-Jun. Mol Cell Oncol. 2014. PMID: 27308329 Free PMC article. Review.
-
SUMO-mediated recruitment allows timely function of the Yen1 nuclease in mitotic cells.PLoS Genet. 2022 Mar 25;18(3):e1009860. doi: 10.1371/journal.pgen.1009860. eCollection 2022 Mar. PLoS Genet. 2022. PMID: 35333860 Free PMC article.
-
SUMO Interacting Motifs: Structure and Function.Cells. 2021 Oct 21;10(11):2825. doi: 10.3390/cells10112825. Cells. 2021. PMID: 34831049 Free PMC article. Review.
-
G1-Cyclin2 (Cln2) promotes chromosome hypercondensation in eco1/ctf7 rad61 null cells during hyperthermic stress in Saccharomyces cerevisiae.G3 (Bethesda). 2022 Jul 29;12(8):jkac157. doi: 10.1093/g3journal/jkac157. G3 (Bethesda). 2022. PMID: 35736360 Free PMC article.
-
The Mgs1/WRNIP1 ATPase is required to prevent a recombination salvage pathway at damaged replication forks.Sci Adv. 2020 Apr 8;6(15):eaaz3327. doi: 10.1126/sciadv.aaz3327. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32285001 Free PMC article.
References
-
- Aroya SB, Kupiec M (2005) The Elg1 replication factor C-like complex: a novel guardian of genome stability. DNA Repair (Amst) 4: 409–417 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

