A local autocrine axis in the testes that regulates spermatogenesis
- PMID: 20571538
- PMCID: PMC4080676
- DOI: 10.1038/nrendo.2010.71
A local autocrine axis in the testes that regulates spermatogenesis
Abstract
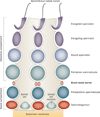
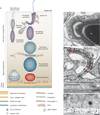
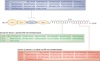
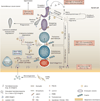
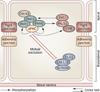
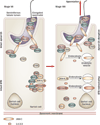
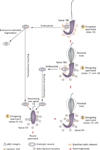
Spermiation--the release of mature spermatozoa from Sertoli cells into the seminiferous tubule lumen--occurs by the disruption of an anchoring device known as the apical ectoplasmic specialization (apical ES). At the same time, the blood-testis barrier (BTB) undergoes extensive restructuring to facilitate the transit of preleptotene spermatocytes. While these two cellular events take place at opposite ends of the Sertoli cell epithelium, the events are in fact tightly coordinated, as any disruption in either process will lead to infertility. A local regulatory axis exists between the apical ES and the BTB in which biologically active laminin fragments produced at the apical ES by the action of matrix metalloproteinase 2 can regulate BTB restructuring directly or indirectly via the hemidesmosome. Equally important, polarity proteins play a crucial part in coordinating cellular events within this apical ES-BTB-hemidesmosome axis. Additionally, testosterone and cytokines work in concert to facilitate BTB restructuring, which enables the transit of spermatocytes while maintaining immunological barrier function. Herein, we will discuss this important autocrine-based cellular axis that parallels the hormonal-based hypothalamic-pituitary-testicular axis that regulates spermatogenesis. This local regulatory axis is the emerging target for male contraception.
Figures
References
-
- Sharpe RM. In: The Physiology of Reproduction. Knobil E, Neill JD, editors. New York: Raven Press; 1994. pp. 1363–1434.
-
- Sokol RZ. Endocrinology of male infertility: evaluation and treatment. Semin. Reprod. Med. 2009;27:149–158. - PubMed
-
- Kakar SS, Malik MT, Winters SJ, Mazhawidza W. Gonadotropin-releasing hormone receptors: structure, expression, and signaling transduction. Vitam. Horm. 2004;69:151–207. - PubMed
-
- McLachlan RI, et al. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J. Androl. 2002;23:149–162. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

