ATP-sensitive K(+) channel-deficient dilated cardiomyopathy proteome remodeled by embryonic stem cell therapy
- PMID: 20572010
- PMCID: PMC3129996
- DOI: 10.1002/stem.465
ATP-sensitive K(+) channel-deficient dilated cardiomyopathy proteome remodeled by embryonic stem cell therapy
Abstract
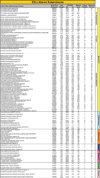
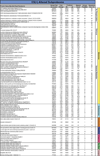
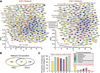
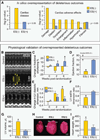
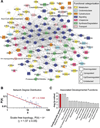
Transplantation of pluripotent stem cells has proven beneficial in heart failure, yet the proteomic landscape underlying repair remains largely uncharacterized. In a genetic model of dilated cardiomyopathy elicited by pressure overload in the KCNJ11 (potassium inwardly rectifying channel, subfamily J, member 11) null mutant, proteome-wide profiles were here resolved by means of a systems approach prior to and following disease manifestation in the absence or presence of embryonic stem cell treatment. Comparative two-dimensional gel electrophoresis revealed a unique cardiomyopathic proteome in the absence of therapy, remodeled in response to stem cell treatment. Specifically, linear ion trap quadrupole-Orbitrap mass spectrometry determined the identities of 93 and 109 differentially expressed proteins from treated and untreated cardiomyopathic hearts, respectively. Mapped protein-protein relationships and corresponding neighborhoods incorporated the stem cell-dependent subproteome into a nonstochastic network with divergent composition from the stem cell-independent counterpart. Stem cell intervention produced a distinct proteome signature across a spectrum of biological processes ranging from energetic metabolism, oxidoreductases, and stress-related chaperones to processes supporting protein synthesis/degradation, signaling, and transport regulation, cell structure and scaffolding. In the absence of treatment, bioinformatic interrogation of the disease-only proteome network prioritized adverse cardiac outcomes, ablated or ameliorated following stem cell transplantation. Functional and structural measurements validated improved myocardial contractile performance, reduced ventricular size and decreased cardiac damage in the treated cohort. Unbiased systems assessment unmasked "cardiovascular development" as a prioritized biological function in stem cell-reconstructed cardiomyopathic hearts. Thus, embryonic stem cell treatment transformed the cardiomyopathic proteome to demote disease-associated adverse effects and sustain a procardiogenic developmental response, supplying a regenerative substrate for heart failure repair.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


References
-
- Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. - PubMed
-
- Hershberger RE, Lindenfeld J, Mestroni L, et al. Genetic evaluation of cardiomyopathy: A Heart Failure Society of America practice guideline. J Card Fail. 2009;15:83–97. - PubMed
-
- Luk A, Ahn E, Soor GS, et al. Dilated cardiomyopathy: A review. J Clin Pathol. 2009;62:219–225. - PubMed
-
- Chien KR. Genomic circuits and the integrative biology of cardiac diseases. Nature. 2000;407:227–232. - PubMed
-
- Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

