The structure and evolution of the murine inhibitor of carbonic anhydrase: a member of the transferrin superfamily
- PMID: 20572014
- PMCID: PMC2975126
- DOI: 10.1002/pro.439
The structure and evolution of the murine inhibitor of carbonic anhydrase: a member of the transferrin superfamily
Abstract
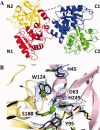
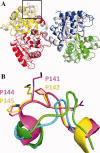
The original signature of the transferrin (TF) family of proteins was the ability to bind ferric iron with high affinity in the cleft of each of two homologous lobes. However, in recent years, new family members that do not bind iron have been discovered. One new member is the inhibitor of carbonic anhydrase (ICA), which as its name indicates, binds to and strongly inhibits certain isoforms of carbonic anhydrase. Recently, mouse ICA has been expressed as a recombinant protein in a mammalian cell system. Here, we describe the 2.4 Å structure of mouse ICA from a pseudomerohedral twinned crystal. As predicted, the structure is bilobal, comprised of two α-β domains per lobe typical of the other family members. As with all but insect TFs, the structure includes the unusual reverse γ-turn in each lobe. The structure is consistent with the fact that introduction of two mutations in the N-lobe of murine ICA (mICA) (W124R and S188Y) allowed it to bind iron with high affinity. Unexpectedly, both lobes of the mICA were found in the closed conformation usually associated with presence of iron in the cleft, and making the structure most similar to diferric pig TF. Two new ICA family members (guinea pig and horse) were identified from genomic sequences and used in evolutionary comparisons. Additionally, a comparison of selection pressure (dN/dS) on functional residues reveals some interesting insights into the evolution of the TF family including that the N-lobe of lactoferrin may be in the process of eliminating its iron binding function.
Copyright © 2010 The Protein Society.
Figures

Similar articles
-
Evolution reversed: the ability to bind iron restored to the N-lobe of the murine inhibitor of carbonic anhydrase by strategic mutagenesis.Biochemistry. 2008 Sep 16;47(37):9847-55. doi: 10.1021/bi801133d. Epub 2008 Aug 20. Biochemistry. 2008. PMID: 18712936 Free PMC article.
-
A novel murine protein with no effect on iron homoeostasis is homologous with transferrin and is the putative inhibitor of carbonic anhydrase.Biochem J. 2007 Aug 15;406(1):85-95. doi: 10.1042/BJ20070384. Biochem J. 2007. PMID: 17511619 Free PMC article.
-
Evolution of the transferrin family: conservation of residues associated with iron and anion binding.Comp Biochem Physiol B Biochem Mol Biol. 2005 Oct;142(2):129-41. doi: 10.1016/j.cbpb.2005.07.007. Comp Biochem Physiol B Biochem Mol Biol. 2005. PMID: 16111909 Review.
-
Ligand variation in the transferrin family: the crystal structure of the H249Q mutant of the human transferrin N-lobe as a model for iron binding in insect transferrins.Biochemistry. 2001 Oct 2;40(39):11670-5. doi: 10.1021/bi010907p. Biochemistry. 2001. PMID: 11570867
-
Lactoferrin and iron: structural and dynamic aspects of binding and release.Biometals. 2004 Jun;17(3):209-16. doi: 10.1023/b:biom.0000027694.40260.70. Biometals. 2004. PMID: 15222467 Review.
Cited by
-
Evolutionary diversification of the vertebrate transferrin multi-gene family.Immunogenetics. 2014 Nov;66(11):651-61. doi: 10.1007/s00251-014-0798-x. Epub 2014 Aug 22. Immunogenetics. 2014. PMID: 25142446 Free PMC article.
-
Prenatal Endotoxin Exposure Induces Fetal and Neonatal Renal Inflammation via Innate and Th1 Immune Activation in Preterm Pigs.Front Immunol. 2020 Sep 30;11:565484. doi: 10.3389/fimmu.2020.565484. eCollection 2020. Front Immunol. 2020. PMID: 33193334 Free PMC article.
-
High-density chemical cross-linking for modeling protein interactions.Proc Natl Acad Sci U S A. 2020 Jan 7;117(1):93-102. doi: 10.1073/pnas.1902931116. Epub 2019 Dec 17. Proc Natl Acad Sci U S A. 2020. PMID: 31848235 Free PMC article.
-
Transferrin-mediated cellular iron delivery.Curr Top Membr. 2012;69:3-35. doi: 10.1016/B978-0-12-394390-3.00001-X. Curr Top Membr. 2012. PMID: 23046645 Free PMC article. Review.
-
Protein expression of the tear film of domestic cats before and after inoculation with Toxoplasma gondii.BMC Vet Res. 2021 Dec 14;17(1):386. doi: 10.1186/s12917-021-03080-9. BMC Vet Res. 2021. PMID: 34906132 Free PMC article.
References
-
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. - PubMed
-
- Mason AB, Everse SJ. Iron transport by transferrin. In: Fuchs H, editor. Iron metabolism and disease. Kerala, India: Research Signpost; 2008. pp. 83–123.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

