Disease resistance in the drywood termite, Incisitermes schwarzi: does nesting ecology affect immunocompetence?
- PMID: 20572790
- PMCID: PMC3014820
- DOI: 10.1673/031.010.4401
Disease resistance in the drywood termite, Incisitermes schwarzi: does nesting ecology affect immunocompetence?
Abstract
Termites live in nests that can differ in microbial load and thus vary in degree of disease risk. It was hypothesized that termite investment in immune response would differ in species living in nest environments that vary in the richness and abundance of microbes. Using the drywood termite, Incisitermes schwarzi Banks (Isoptera: Kalotermitidae), as a model for species having low nest and cuticular microbial loads, the susceptibility of individuals and groups to conidia of the entomopathogenic fungus, Metarhizium anisopliae Sorokin (Hypocreales: Clavicipitaceae), was examined. The survivorship of I. schwarzi was compared to that of the dampwood termite, Zootermopsis angusticollis Hagen (Termopsidae), a species with comparatively high microbial loads. The results indicated that I. schwarzi derives similar benefits from group living as Z. angusticollis: isolated termites had 5.5 times the hazard ratio of death relative to termites nesting in groups of 25 while termites in groups of 10 did not differ significantly from the groups of 25. The results also indicated, after controlling for the influence of group size and conidia exposure on survivorship, that Z. angusticollis was significantly more susceptible to fungal infection than I. schwarzi, the former having 1.6 times the hazard ratio of death relative to drywood termites. Thus, disease susceptibility and individual investment in immunocompetence may not be dependent on interspecific variation in microbial pressures. The data validate prior studies indicating that sociality has benefits in infection control and suggest that social mechanisms of disease resistance, rather than individual physiological and immunological adaptations, may have been the principle target of selection related to variation in infection risk from microbes in the nest environment of different termite species.
Figures
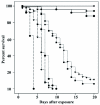
 ), groups of 10 (squares
), groups of 10 (squares  ), or groups
of 25 (inverted triangles
), or groups
of 25 (inverted triangles  ) following exposure to a low (
) following exposure to a low ( ) or high (
) or high ( ) dose of conidia/ml, or control (
) dose of conidia/ml, or control ( ). High
quality figures are available online.
). High
quality figures are available online.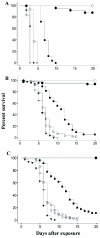
 ) or high (
) or high ( ) dose of conidia. Control: (
) dose of conidia. Control: ( ).
).References
-
- Abe T. Kawano S, Connel JH, Hidaka T. Evolution and Coadaptation in Biotic Communities. University of Tokyo Press; 1987. Evolution of life types in termites. pp. 125–148.
-
- Armitage SAO, Thompson JJW, Rolff J, Siva-Jothy MT. Examining costs of induced and constitutive immune investment in Tenebrio molitor. Journal of Evolutionary Biology. 2003;16:1038–1044. - PubMed
-
- Boots M, Begon M. Trade-offs with resistance to a granulosis virus in the Indian meal moth, examined by a laboratory evolution experiment. Functional Ecology. 1993;7:528–534.
-
- Bulmer MS, Crozier RH. Duplication and diversifying selection among termite antifungal peptides. Molecular Biology and Evolution. 2004;21:2256–2264. - PubMed
-
- Calleri DV, II, Reid EM, Rosengaus RB, Vargo EL, Traniello JFA. Inbreeding and disease resistance in a social insect: Effects of genetic variation on immunocompetence in the termite Zootermopsis angusticollis. Proceedings of the Royal Society of London Series B, Biological Sciences. 2006;273:2633–2640. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

