The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway
- PMID: 20573068
- PMCID: PMC2919637
- DOI: 10.1111/j.1600-0854.2010.01089.x
The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway
Abstract
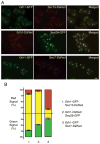
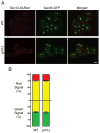
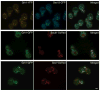
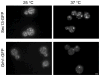
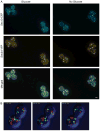
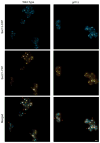
In mammalian cells, the 'Golgi reassembly and stacking protein' (GRASP) family has been implicated in Golgi stacking, but the broader functions of GRASP proteins are still unclear. The yeast Saccharomyces cerevisiae contains a single non-essential GRASP homolog called Grh1. However, Golgi cisternae in S. cerevisiae are not organized into stacks, so a possible structural role for Grh1 has been difficult to test. Here, we examined the localization and function of Grh1 in S. cerevisiae and in the related yeast Pichia pastoris, which has stacked Golgi cisternae. In agreement with earlier studies indicating that Grh1 interacts with coat protein II (COPII) vesicle coat proteins, we find that Grh1 colocalizes with COPII at transitional endoplasmic reticulum (tER) sites in both yeasts. Deletion of P. pastoris Grh1 had no obvious effect on the structure of tER-Golgi units. To test the role of S. cerevisiae Grh1, we exploited the observation that inhibiting ER export in S. cerevisiae generates enlarged tER sites that are often associated with the cis Golgi. This tER-Golgi association was preserved in the absence of Grh1. The combined data suggest that Grh1 acts early in the secretory pathway, but is dispensable for the organization of secretory compartments.
Figures


Similar articles
-
Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae.J Cell Biol. 1999 Apr 5;145(1):69-81. doi: 10.1083/jcb.145.1.69. J Cell Biol. 1999. PMID: 10189369 Free PMC article.
-
The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic.J Cell Biol. 2007 Jan 29;176(3):255-61. doi: 10.1083/jcb.200607151. J Cell Biol. 2007. PMID: 17261844 Free PMC article.
-
Remodeling of secretory compartments creates CUPS during nutrient starvation.J Cell Biol. 2014 Dec 22;207(6):695-703. doi: 10.1083/jcb.201407119. Epub 2014 Dec 15. J Cell Biol. 2014. PMID: 25512390 Free PMC article.
-
Vesicle-mediated export from the ER: COPII coat function and regulation.Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review.
-
Making COPII coats.Cell. 2007 Jun 29;129(7):1251-2. doi: 10.1016/j.cell.2007.06.015. Cell. 2007. PMID: 17604713 Review.
Cited by
-
Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation.Trends Cell Biol. 2012 Aug;22(8):397-406. doi: 10.1016/j.tcb.2012.04.008. Epub 2012 Jun 5. Trends Cell Biol. 2012. PMID: 22677446 Free PMC article. Review.
-
Membrane adhesion dictates Golgi stacking and cisternal morphology.Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):1849-54. doi: 10.1073/pnas.1323895111. Epub 2014 Jan 21. Proc Natl Acad Sci U S A. 2014. PMID: 24449908 Free PMC article.
-
Sec16 influences transitional ER sites by regulating rather than organizing COPII.Mol Biol Cell. 2013 Nov;24(21):3406-19. doi: 10.1091/mbc.E13-04-0185. Epub 2013 Sep 4. Mol Biol Cell. 2013. PMID: 24006484 Free PMC article.
-
Integrated self-organization of transitional ER and early Golgi compartments.Bioessays. 2014 Feb;36(2):129-33. doi: 10.1002/bies.201300131. Epub 2013 Nov 18. Bioessays. 2014. PMID: 24242672 Free PMC article.
-
COPI selectively drives maturation of the early Golgi.Elife. 2015 Dec 28;4:e13232. doi: 10.7554/eLife.13232. Elife. 2015. PMID: 26709839 Free PMC article.
References
-
- Mowbrey K, Dacks JB. Evolution and diversity of the Golgi body. FEBS Lett. 2009;583:3738–3745. - PubMed
-
- Cluett EB, Brown WJ. Adhesion of Golgi cisternae by proteinaceous interactions: intercisternal bridges as putative adhesive structures. J Cell Sci. 1992;103:773–784. - PubMed
-
- Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. - PubMed
-
- Ramirez IB, Lowe M. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol. 2009;20:770–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

