Fucose-deficient hematopoietic stem cells have decreased self-renewal and aberrant marrow niche occupancy
- PMID: 20573072
- PMCID: PMC2946970
- DOI: 10.1111/j.1537-2995.2010.02745.x
Fucose-deficient hematopoietic stem cells have decreased self-renewal and aberrant marrow niche occupancy
Abstract
Background: Modification of Notch receptors by O-linked fucose and its further elongation by the Fringe family of glycosyltransferase has been shown to be important for Notch signaling activation. Our recent studies disclose a myeloproliferative phenotype, hematopoietic stem cell (HSC) dysfunction, and abnormal Notch signaling in mice deficient in FX, which is required for fucosylation of a number of proteins including Notch. The purpose of this study was to assess the self-renewal and stem cell niche features of fucose-deficient HSCs.
Study design and methods: Homeostasis and maintenance of HSCs derived from FX(-/-) mice were studied by serial bone marrow transplantation, homing assay, and cell cycle analysis. Two-photon intravital microscopy was performed to visualize and compare the in vivo marrow niche occupancy by fucose-deficient and wild-type (WT) HSCs.
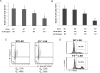
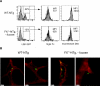
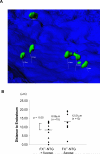
Results: Marrow progenitors from FX(-/-) mice had mild homing defects that could be partially prevented by exogenous fucose supplementation. Fucose-deficient HSCs from FX(-/-) mice displayed decreased self-renewal capability compared with the WT controls. This is accompanied with their increased cell cycling activity and suppressed Notch ligand binding. When tracked in vivo by two-photon intravital imaging, the fucose-deficient HSCs were found localized farther from the endosteum of the calvarium marrow than the WT HSCs.
Conclusions: The current reported aberrant niche occupancy by HSCs from FX(-/-) mice, in the context of a faulty blood lineage homeostasis and HSC dysfunction in mice expressing Notch receptors deficient in O-fucosylation, suggests that fucosylation-modified Notch receptor may represent a novel extrinsic regulator for HSC engraftment and HSC niche maintenance.
© 2010 American Association of Blood Banks.
Figures
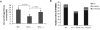
Similar articles
-
Myeloproliferation and hematopoietic stem cell dysfunction due to defective Notch receptor modification by O-fucose glycans.Semin Immunopathol. 2012 May;34(3):455-69. doi: 10.1007/s00281-012-0303-2. Epub 2012 Mar 14. Semin Immunopathol. 2012. PMID: 22415200 Review.
-
The endoplasmic reticulum chaperone protein GRP94 is required for maintaining hematopoietic stem cell interactions with the adult bone marrow niche.PLoS One. 2011;6(5):e20364. doi: 10.1371/journal.pone.0020364. Epub 2011 May 24. PLoS One. 2011. PMID: 21647226 Free PMC article.
-
Secretory cell hyperplasia and defects in Notch activity in a mouse model of leukocyte adhesion deficiency type II.Gastroenterology. 2010 Mar;138(3):1079-90.e1-5. doi: 10.1053/j.gastro.2009.10.049. Epub 2009 Nov 10. Gastroenterology. 2010. PMID: 19900444
-
Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver.Blood. 2010 Jul 29;116(4):544-53. doi: 10.1182/blood-2009-08-240903. Epub 2010 May 4. Blood. 2010. PMID: 20442369
-
Notch signaling and the bone marrow hematopoietic stem cell niche.Bone. 2010 Feb;46(2):281-5. doi: 10.1016/j.bone.2009.08.007. Epub 2009 Aug 11. Bone. 2010. PMID: 19679213 Free PMC article. Review.
Cited by
-
Myeloproliferation and hematopoietic stem cell dysfunction due to defective Notch receptor modification by O-fucose glycans.Semin Immunopathol. 2012 May;34(3):455-69. doi: 10.1007/s00281-012-0303-2. Epub 2012 Mar 14. Semin Immunopathol. 2012. PMID: 22415200 Review.
-
The transcription factor ATF2 promotes melanoma metastasis by suppressing protein fucosylation.Sci Signal. 2015 Dec 8;8(406):ra124. doi: 10.1126/scisignal.aac6479. Sci Signal. 2015. PMID: 26645581 Free PMC article.
-
Notch Receptor-Ligand Engagement Maintains Hematopoietic Stem Cell Quiescence and Niche Retention.Stem Cells. 2015 Jul;33(7):2280-93. doi: 10.1002/stem.2031. Epub 2015 May 13. Stem Cells. 2015. PMID: 25851125 Free PMC article.
-
Direct Visualization of Live Zebrafish Glycans via Single-Step Metabolic Labeling with Fluorophore-Tagged Nucleotide Sugars.Angew Chem Int Ed Engl. 2019 Oct 1;58(40):14327-14333. doi: 10.1002/anie.201907410. Epub 2019 Aug 28. Angew Chem Int Ed Engl. 2019. PMID: 31295389 Free PMC article. Review.
-
A Review of Advances in Hematopoietic Stem Cell Mobilization and the Potential Role of Notch2 Blockade.Cell Transplant. 2020 Jan-Dec;29:963689720947146. doi: 10.1177/0963689720947146. Cell Transplant. 2020. PMID: 32749152 Free PMC article. Review.
References
-
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. - PubMed
-
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

