Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias
- PMID: 20573078
- PMCID: PMC2856043
- DOI: 10.1111/j.1365-2125.2010.03629.x
Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias
Abstract
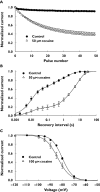
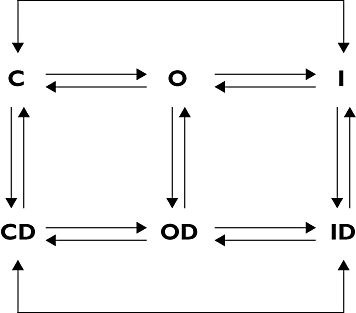
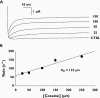
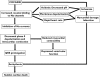
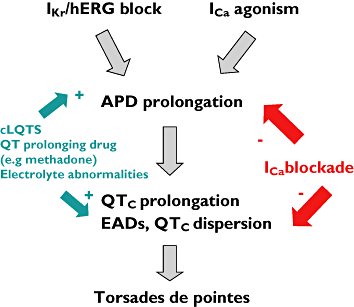
Cocaine is a highly active stimulant that alters dopamine metabolism in the central nervous system resulting in a feeling of euphoria that with time can lead to addictive behaviours. Cocaine has numerous deleterious effects in humans including seizures, vasoconstriction, ischaemia, increased heart rate and blood pressure, cardiac arrhythmias and sudden death. The cardiotoxic effects of cocaine are indirectly mediated by an increase in sympathomimetic stimulation to the heart and coronary vasculature and by a direct effect on the ion channels responsible for maintaining the electrical excitability of the heart. The direct and indirect effects of cocaine work in tandem to disrupt the co-ordinated electrical activity of the heart and have been associated with life-threatening cardiac arrhythmias. This review focuses on the direct effects of cocaine on cardiac ion channels, with particular focus on sodium, potassium and calcium channels, and on the contributions of these channels to cocaine-induced arrhythmias. Companion articles in this edition of the journal examine the epidemiology of cocaine use (Wood & Dargan) and the treatment of cocaine-associated arrhythmias (Hoffmann).
Figures

Similar articles
-
Treatment of patients with cocaine-induced arrhythmias: bringing the bench to the bedside.Br J Clin Pharmacol. 2010 May;69(5):448-57. doi: 10.1111/j.1365-2125.2010.03632.x. Br J Clin Pharmacol. 2010. PMID: 20573080 Free PMC article. Review.
-
Putting cocaine use and cocaine-associated cardiac arrhythmias into epidemiological and clinical perspective.Br J Clin Pharmacol. 2010 May;69(5):443-7. doi: 10.1111/j.1365-2125.2010.03630.x. Br J Clin Pharmacol. 2010. PMID: 20573079 Free PMC article.
-
Cocaine: a review of its toxic actions on cardiac function.Crit Rev Toxicol. 1995;25(2):113-32. doi: 10.3109/10408449509021610. Crit Rev Toxicol. 1995. PMID: 7612173 Review.
-
Stimulant Drugs of Abuse and Cardiac Arrhythmias.Circ Arrhythm Electrophysiol. 2022 Jan;15(1):e010273. doi: 10.1161/CIRCEP.121.010273. Epub 2021 Dec 28. Circ Arrhythm Electrophysiol. 2022. PMID: 34961335 Free PMC article. Review.
-
Management of cocaine-induced cardiac arrhythmias due to cardiac ion channel dysfunction.Clin Toxicol (Phila). 2009 Jan;47(1):14-23. doi: 10.1080/15563650802339373. Clin Toxicol (Phila). 2009. PMID: 18815938 Review.
Cited by
-
Brugada Phenotype Following a Cocaine Overdose.Cureus. 2024 Jul 4;16(7):e63861. doi: 10.7759/cureus.63861. eCollection 2024 Jul. Cureus. 2024. PMID: 39099899 Free PMC article.
-
Synthetic cannabinoids and potential cardiac arrhythmia risk: an important message for drug users.Ther Adv Drug Saf. 2020 Mar 24;11:2042098620913416. doi: 10.1177/2042098620913416. eCollection 2020. Ther Adv Drug Saf. 2020. PMID: 32269749 Free PMC article. No abstract available.
-
Cocaine Reduces Ciliary Beat Frequency of Human Nasal Epithelial Cells.In Vivo. 2020 Nov-Dec;34(6):3285-3289. doi: 10.21873/invivo.12166. In Vivo. 2020. PMID: 33144435 Free PMC article.
-
The Impact of Potent Addictive Substances on Angiogenic Behavior: A Comprehensive Review.Curr Neuropharmacol. 2025;23(5):511-523. doi: 10.2174/1570159X23666240905125037. Curr Neuropharmacol. 2025. PMID: 39248059 Free PMC article. Review.
-
Cellular and molecular responses to acute cocaine treatment in neuronal-like N2a cells: potential mechanism for its resistance in cell death.Cell Death Discov. 2018 Jul 17;4:13. doi: 10.1038/s41420-018-0078-x. eCollection 2018. Cell Death Discov. 2018. PMID: 30210816 Free PMC article.
References
-
- Fischman MW, Schuster CR, Resnekov L, Shick JF, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry. 1976;33:983–9. - PubMed
-
- Billman GE. Mechanisms responsible for the cardiotoxic effects of cocaine. FASEB J. 1990;4:2469–75. - PubMed
-
- Isner JM, Chokshi SK. Cardiovascular complications of cocaine. Curr Probl Cardiol. 1991;16:89–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

