The Drosophila Anion Exchanger (DAE) lacks a detectable interaction with the spectrin cytoskeleton
- PMID: 20573195
- PMCID: PMC2901199
- DOI: 10.1186/1477-5751-9-5
The Drosophila Anion Exchanger (DAE) lacks a detectable interaction with the spectrin cytoskeleton
Abstract
Background: Current models suggest that the spectrin cytoskeleton stabilizes interacting ion transport proteins at the plasma membrane. The human erythrocyte anion exchanger (AE1) was the first membrane transport protein found to be associated with the spectrin cytoskeleton. Here we evaluated a conserved anion exchanger from Drosophila (DAE) as a marker for studies of the downstream effects of spectrin cytoskeleton mutations.
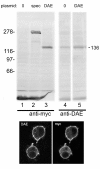
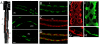
Results: Sequence comparisons established that DAE belongs to the SLC4A1-3 subfamily of anion exchangers that includes human AE1. Striking sequence conservation was observed in the C-terminal membrane transport domain and parts of the N-terminal cytoplasmic domain, but not in the proposed ankyrin-binding site. Using an antibody raised against DAE and a recombinant transgene expressed in Drosophila S2 cells DAE was shown to be a 136 kd plasma membrane protein. A major site of expression was found in the stomach acid-secreting region of the larval midgut. DAE codistributed with an infolded subcompartment of the basal plasma membrane of interstitial cells. However, spectrin did not codistribute with DAE at this site or in anterior midgut cells that abundantly expressed both spectrin and DAE. Ubiquitous knockdown of DAE with dsRNA eliminated antibody staining and was lethal, indicating that DAE is an essential gene product in Drosophila.
Conclusions: Based on the lack of colocalization and the lack of sequence conservation at the ankyrin-binding site, it appears that the well-characterized interaction between AE1 and the spectrin cytoskeleton in erythrocytes is not conserved in Drosophila. The results establish a pattern in which most of the known interactions between the spectrin cytoskeleton and the plasma membrane in mammals do not appear to be conserved in Drosophila.
Figures



References
-
- Lux SE, Palek J. In: Blood: Principles and practice of hematology. Handin RI, Lux SE, Stossel TP, editor. Philadelphia: J.B. Lippincott Co.; 1995. Disorders of the Red Cell Membrane; pp. 1701–1818.
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol Rev. 2001. pp. 1353–1388. - PubMed
-
- Dubreuil RR. In: Advance in Molecular Cell Biology. Khurana S, editor. Vol. 37. New York:Elsevier; 2006. Spectrin function: A survey of genetic systems from Drosophila to humans; pp. 68–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

