Effect of high intratesticular estrogen on global gene expression and testicular cell number in rats
- PMID: 20573204
- PMCID: PMC2906496
- DOI: 10.1186/1477-7827-8-72
Effect of high intratesticular estrogen on global gene expression and testicular cell number in rats
Abstract
Background: The identification of estrogen receptors alpha and beta and aromatase in the testis has highlighted the important role of estrogens in regulating spermatogenesis. There is a wealth of information on the deleterious effects of fetal and neonatal exposure of estrogens and xenoestrogens in the testis, including spermiation failure and germ cell apoptosis. However, very little is known about gene transcripts affected by exogenous estradiol exposure in the testis. The objective of the present study was to unveil global gene expression profiles and testicular cell number changes in rats after estradiol treatment.
Methods: 17beta-estradiol was administered to adult male rats at a dose of 100 micrograms/kg body weight in saline daily for 10 days; male rats receiving only saline were used as controls. Microarray analysis was performed to examine global gene expression profiles with or without estradiol treatment. Real time RT-PCR was conducted to verify the microarray data. In silico promoter and estrogen responsive elements (EREs) analysis was carried out for the differentially expressed genes in response to estradiol. Quantitation of testicular cell number based on ploidy was also performed using flow cytometry in rats with or without estradiol treatment.
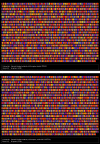
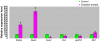
Results: We found that 221 genes and expressed sequence tags (ESTs) were differentially expressed in rat testes treated with estradiol compared to the control; the microarray data were confirmed by real time RT-PCR. Gene Ontology analysis revealed that a number of the differentially expressed genes are involved in androgen and xenobiotic metabolism, maintenance of cell cytoskeleton, endocytosis, and germ cell apoptosis. A total of 33 up-regulated genes and 67 down-regulated genes showed the presence of EREs. Flow cytometry showed that estradiol induced a significant decrease in 2n cells (somatic and germ cells) and 4n cells (pachytene spermatocytes) and a marked increase in the number of elongated and elongating spermatids.
Conclusions: This study provides a novel insight into the molecular basis for spermiation failure and apoptosis caused by 17beta-estradiol and it also offers new mechanisms by which adult exposure to environmental estrogens can affect spermatogenesis and fertility.
Figures
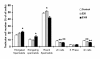
References
-
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl. 2000;21:107–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

