Detection and analysis of alternative splicing in Yarrowia lipolytica reveal structural constraints facilitating nonsense-mediated decay of intron-retaining transcripts
- PMID: 20573210
- PMCID: PMC2911113
- DOI: 10.1186/gb-2010-11-6-r65
Detection and analysis of alternative splicing in Yarrowia lipolytica reveal structural constraints facilitating nonsense-mediated decay of intron-retaining transcripts
Abstract
Background: Hemiascomycetous yeasts have intron-poor genomes with very few cases of alternative splicing. Most of the reported examples result from intron retention in Saccharomyces cerevisiae and some have been shown to be functionally significant. Here we used transcriptome-wide approaches to evaluate the mechanisms underlying the generation of alternative transcripts in Yarrowia lipolytica, a yeast highly divergent from S. cerevisiae.
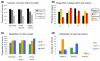
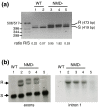
Results: Experimental investigation of Y. lipolytica gene models identified several cases of alternative splicing, mostly generated by intron retention, principally affecting the first intron of the gene. The retention of introns almost invariably creates a premature termination codon, as a direct consequence of the structure of intron boundaries. An analysis of Y. lipolytica introns revealed that introns of multiples of three nucleotides in length, particularly those without stop codons, were underrepresented. In other organisms, premature termination codon-containing transcripts are targeted for degradation by the nonsense-mediated mRNA decay (NMD) machinery. In Y. lipolytica, homologs of S. cerevisiae UPF1 and UPF2 genes were identified, but not UPF3. The inactivation of Y. lipolytica UPF1 and UPF2 resulted in the accumulation of unspliced transcripts of a test set of genes.
Conclusions: Y. lipolytica is the hemiascomycete with the most intron-rich genome sequenced to date, and it has several unusual genes with large introns or alternative transcription start sites, or introns in the 5' UTR. Our results suggest Y. lipolytica intron structure is subject to significant constraints, leading to the under-representation of stop-free introns. Consequently, intron-containing transcripts are degraded by a functional NMD pathway.
Figures





Similar articles
-
Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon.Genes Dev. 1995 Feb 15;9(4):423-36. doi: 10.1101/gad.9.4.423. Genes Dev. 1995. PMID: 7883167
-
Alternative splicing regulates targeting of malate dehydrogenase in Yarrowia lipolytica.DNA Res. 2012 Jun;19(3):231-44. doi: 10.1093/dnares/dss007. Epub 2012 Feb 24. DNA Res. 2012. PMID: 22368181 Free PMC article.
-
Large-scale evidence for conservation of NMD candidature across mammals.PLoS One. 2010 Jul 21;5(7):e11695. doi: 10.1371/journal.pone.0011695. PLoS One. 2010. PMID: 20657786 Free PMC article.
-
The intronome of budding yeasts.C R Biol. 2011 Aug-Sep;334(8-9):662-70. doi: 10.1016/j.crvi.2011.05.015. Epub 2011 Jul 6. C R Biol. 2011. PMID: 21819948 Review.
-
Intron retention in mRNA: No longer nonsense: Known and putative roles of intron retention in normal and disease biology.Bioessays. 2016 Jan;38(1):41-9. doi: 10.1002/bies.201500117. Epub 2015 Nov 27. Bioessays. 2016. PMID: 26612485 Review.
Cited by
-
FgPrp4 Kinase Is Important for Spliceosome B-Complex Activation and Splicing Efficiency in Fusarium graminearum.PLoS Genet. 2016 Apr 8;12(4):e1005973. doi: 10.1371/journal.pgen.1005973. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27058959 Free PMC article.
-
Error prevention and mitigation as forces in the evolution of genes and genomes.Nat Rev Genet. 2011 Nov 18;12(12):875-81. doi: 10.1038/nrg3092. Nat Rev Genet. 2011. PMID: 22094950 Review.
-
A molecular genetic toolbox for Yarrowia lipolytica.Biotechnol Biofuels. 2017 Jan 3;10:2. doi: 10.1186/s13068-016-0687-7. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28066508 Free PMC article.
-
Biotransformation of acetophenone and its halogen derivatives by Yarrowia lipolytica strains.Ann Microbiol. 2015;65(2):1097-1107. doi: 10.1007/s13213-014-0955-3. Epub 2014 Aug 22. Ann Microbiol. 2015. PMID: 26005401 Free PMC article.
-
Alternative splicing acting as a bridge in evolution.Stem Cell Investig. 2015 Oct 30;2:19. doi: 10.3978/j.issn.2306-9759.2015.10.01. eCollection 2015. Stem Cell Investig. 2015. PMID: 27358887 Free PMC article.
References
-
- Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, Goffard N, Frangeul L, Aigle M, Anthouard V, Babour A, Barbe V, Barnay S, Blanchin S, Beckerich JM, Beyne E, Bleykasten C, Boisramé A, Boyer J, Cattolico L, Confanioleri F, De Daruvar A, Despons L, Fabre E, Fairhead C, Ferry-Dumazet H. Genome evolution in yeasts. Nature. 2004;430:35–44. doi: 10.1038/nature02579. - DOI - PubMed
-
- Souciet JL, Dujon B, Gaillardin C, Johnston M, Baret PV, Cliften P, Sherman DJ, Weissenbach J, Westhof E, Wincker P, Jubin C, Poulain J, Barbe V, Ségurens B, Artiguenave F, Anthouard V, Vacherie B, Val ME, Fulton RS, Minx P, Wilson R, Durrens P, Jean G, Marck C, Martin T, Nikolski M, Rolland T, Seret ML, Casarégola S, Despons L. Comparative genomics of protoploid Saccharomycetaceae. Genome Res. 2009;19:1696–1709. doi: 10.1101/gr.091546.109. - DOI - PMC - PubMed
-
- Dietrich FS, Voegeli S, Brachat S, Lerch A, Gates K, Steiner S, Mohr C, Pöhlmann R, Luedi P, Choi S, Wing RA, Flavier A, Gaffney TD, Philippsen P. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science. 2004;304:304–307. doi: 10.1126/science.1095781. - DOI - PubMed
-
- Marck C, Kachouri-Lafond R, Lafontaine I, Westhof E, Dujon B, Grosjean H. The RNA polymerase III-dependent family of genes in hemiascomycetes: comparative RNomics, decoding strategies, transcription and evolutionary implications. Nucleic Acids Res. 2006;34:1816–1835. doi: 10.1093/nar/gkl085. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

