Assessment of mitral bioprostheses using cardiovascular magnetic resonance
- PMID: 20573227
- PMCID: PMC2898806
- DOI: 10.1186/1532-429X-12-36
Assessment of mitral bioprostheses using cardiovascular magnetic resonance
Abstract
Background: The orifice area of mitral bioprostheses provides important information regarding their hemodynamic performance. It is usually calculated by transthoracic echocardiography (TTE), however, accurate and reproducible determination may be challenging. Cardiovascular magnetic resonance (CMR) has been proven as an accurate alternative for assessing aortic bioprostheses. However, whether CMR can be similarly applied for bioprostheses in the mitral position, particularly in the presence of frequently coincident arrhythmias, is unclear. The aim of the study is to test the feasibility of CMR to evaluate the orifice area of mitral bioprostheses.
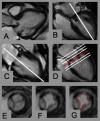
Methods: CMR planimetry was performed in 18 consecutive patients with mitral bioprostheses (n = 13 Hancock(R), n = 4 Labcore(R), n = 1 Perimount(R); mean time since implantation 4.5 +/- 3.9 years) in an imaging plane perpendicular to the transprosthetic flow using steady-state free-precession cine imaging under breath-hold conditions on a 1.5T MR system. CMR results were compared with pressure half-time derived orifice areas obtained by TTE.
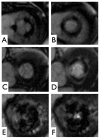
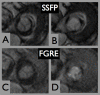
Results: Six subjects were in sinus rhythm, 11 in atrial fibrillation, and 1 exhibited frequent ventricular extrasystoles. CMR image quality was rated as good in 10, moderate in 6, and significantly impaired in 2 subjects. In one prosthetic type (Perimount(R)), strong stent artifacts occurred. Orifice areas by CMR (mean 2.1 +/- 0.3 cm2) and TTE (mean 2.1 +/- 0.3 cm2) correlated significantly (r = 0.94; p < 0.001). Bland-Altman analysis showed a 95% confidence interval from -0.16 to 0.28 cm2 (mean difference 0.06 +/- 0.11 cm2; range -0.1 to 0.3 cm2). Intra- and inter-observer variabilities of CMR planimetry were 4.5 +/- 2.9% and 7.9 +/- 5.2%.
Conclusions: The assessment of mitral bioprostheses using CMR is feasible even in those with arrhythmias, providing orifice areas with close agreement to echocardiography and low observer dependency. Larger samples with a greater variety of prosthetic types and more cases of prosthetic dysfunction are required to confirm these preliminary results.
Figures
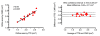
Similar articles
-
Feasibility of cardiovascular magnetic resonance to assess the orifice area of aortic bioprostheses.Circ Cardiovasc Imaging. 2009 Sep;2(5):397-404, 2 p following 404. doi: 10.1161/CIRCIMAGING.108.840967. Epub 2009 May 22. Circ Cardiovasc Imaging. 2009. PMID: 19808628
-
In vitro assessment of heart valve bioprostheses by cardiovascular magnetic resonance: four-dimensional mapping of flow patterns and orifice area planimetry.Eur J Cardiothorac Surg. 2011 Sep;40(3):736-42. doi: 10.1016/j.ejcts.2010.12.040. Epub 2011 Feb 20. Eur J Cardiothorac Surg. 2011. PMID: 21342775
-
Aortic dilatation in patients with prosthetic aortic valve: comparison of MRI and echocardiography.J Heart Valve Dis. 2010 May;19(3):349-56. J Heart Valve Dis. 2010. PMID: 20583398
-
Routine cine-CMR for prosthesis-associated mitral regurgitation: a multicenter comparison to echocardiography.J Heart Valve Dis. 2014 Sep;23(5):575-82. J Heart Valve Dis. 2014. PMID: 25799706 Free PMC article.
-
Quantification of stenotic mitral valve area and diagnostic accuracy of mitral stenosis by dual-source computed tomography in patients with atrial fibrillation: comparison with cardiovascular magnetic resonance and transthoracic echocardiography.Int J Cardiovasc Imaging. 2015 Jun;31 Suppl 1:103-14. doi: 10.1007/s10554-014-0488-7. Epub 2014 Jul 11. Int J Cardiovasc Imaging. 2015. PMID: 25011534
Cited by
-
Review of journal of cardiovascular magnetic resonance 2010.J Cardiovasc Magn Reson. 2011 Sep 13;13(1):48. doi: 10.1186/1532-429X-13-48. J Cardiovasc Magn Reson. 2011. PMID: 21914185 Free PMC article. Review.
-
Functional assessment of bioprosthetic mitral valves by cardiovascular magnetic resonance: An in vitro validation and comparison to Doppler echocardiography.J Cardiovasc Magn Reson. 2020 Jul 30;22(1):55. doi: 10.1186/s12968-020-00635-x. J Cardiovasc Magn Reson. 2020. PMID: 32727590 Free PMC article.
-
Effects of heart valve prostheses on phase contrast flow measurements in Cardiovascular Magnetic Resonance - a phantom study.J Cardiovasc Magn Reson. 2017 Jan 16;19(1):5. doi: 10.1186/s12968-016-0319-1. J Cardiovasc Magn Reson. 2017. PMID: 28088917 Free PMC article.
-
Diagnostic evaluation of left-sided prosthetic heart valve dysfunction.Nat Rev Cardiol. 2011 May 17;8(8):466-78. doi: 10.1038/nrcardio.2011.71. Nat Rev Cardiol. 2011. PMID: 21587215 Review.
-
Star GK Bileaflet Mechanical Valve Prosthesis-Patient Mismatch after Mitral Valve Replacement: A Chinese Multicenter Clinical Study.Med Sci Monit. 2015 Aug 27;21:2542-6. doi: 10.12659/MSM.894044. Med Sci Monit. 2015. PMID: 26313311 Free PMC article.
References
-
- Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA Jr, Nakatani S, Quiñones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M. American Society of Echocardiography's Guidelines and Standards Committee; Task Force on Prosthetic Valves; American College of Cardiology Cardiovascular Imaging Committee; Cardiac Imaging Committee of the American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography; American College of Cardiology Foundation; American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography . Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. quiz 1082-1014. - DOI - PubMed
-
- Pflederer T, Flachskampf FA. Echocardiographic follow-up after heart valve replacement. Heart (British Cardiac Society) pp. 75–85. - PubMed
-
- von Knobelsdorff-Brenkenhoff F, Rudolph A, Wassmuth R, Bohl S, Buschmann EE, Abdel-Aty H, Dietz R, Schulz-Menger J. Feasibility of cardiovascular magnetic resonance to assess the orifice area of aortic bioprostheses. Circ Cardiovasc Imaging. 2009;2:397–404. doi: 10.1161/CIRCIMAGING.108.840967. 392 p following 404. - DOI - PubMed
-
- Lin SJ, Brown PA, Watkins MP, Williams TA, Lehr KA, Liu W, Lanza GM, Wickline SA, Caruthers SD. Quantification of stenotic mitral valve area with magnetic resonance imaging and comparison with Doppler ultrasound. Journal of the American College of Cardiology. 2004;44:133–137. doi: 10.1016/j.jacc.2004.03.038. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

