Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF)
- PMID: 20573231
- PMCID: PMC2898775
- DOI: 10.1186/1465-9921-11-85
Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF)
Abstract
Background: Airway remodelling is thought to be under the control of a complex group of molecules belonging to the transforming growth factor (TGF)-superfamily. The bone morphogenetic proteins (BMPs) belong to this family and have been shown to regulate fibrosis in kidney and liver diseases. However, the role of BMPs in lung remodelling remains unclear. BMPs may regulate tissue remodelling in asthma by controlling TGF-beta-induced profibrotic functions in lung fibroblasts.
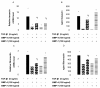
Methods: Cell cultures were exposed to TGF-beta1 alone or in the presence of BMP-4 or BMP-7; control cultures were exposed to medium only. Cell proliferation was assessed by quantification of the incorporation of [3H]-thymidine. The expression of the mRNA encoding collagen type I and IV, tenascin C and fibronectin in normal human lung fibroblasts (NHLF) was determined by real-time quantitative PCR and the main results were confirmed by ELISA. Cell differentiation was determined by the analysis of the expression of alpha-smooth muscle actin (alpha-SMA) by western blot and immunohistochemistry. The effect on matrix metalloproteinase (MMP) activity was assessed by zymography.
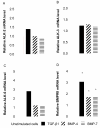
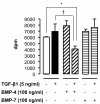
Results: We have demonstrated TGF-beta1 induced upregulation of mRNAs encoding the extracellular matrix proteins, tenascin C, fibronectin and collagen type I and IV when compared to unstimulated NHLF, and confirmed these results at the protein level. BMP-4, but not BMP-7, reduced TGF-beta1-induced extracellular matrix protein production. TGF-beta1 induced an increase in the activity of the pro-form of MMP-2 which was inhibited by BMP-7 but not BMP-4. Both BMP-4 and BMP-7 downregulated TGF-beta1-induced MMP-13 release compared to untreated and TGF-beta1-treated cells. TGF-beta1 also induced a myofibroblast-like transformation which was partially inhibited by BMP-7 but not BMP-4.
Conclusions: Our study suggests that some regulatory properties of BMP-7 may be tissue or cell type specific and unveil a potential regulatory role for BMP-4 in the regulation of lung fibroblast function.
Figures
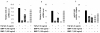


References
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

