First evidence of overlaps between HIV-Associated Dementia (HAD) and non-viral neurodegenerative diseases: proteomic analysis of the frontal cortex from HIV+ patients with and without dementia
- PMID: 20573273
- PMCID: PMC2904315
- DOI: 10.1186/1750-1326-5-27
First evidence of overlaps between HIV-Associated Dementia (HAD) and non-viral neurodegenerative diseases: proteomic analysis of the frontal cortex from HIV+ patients with and without dementia
Abstract
Background: The pathogenesis of HIV-associated dementia (HAD) is poorly understood. To date, detailed proteomic fingerprinting directly from autopsied brain tissues of HAD and HIV non-dementia patients has not been performed.
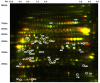
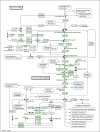
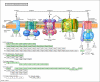
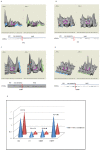
Result: Here, we have analyzed total proteins from the frontal cortex of 9 HAD and 5 HIV non-dementia patients. Using 2-Dimensional differential in-gel electrophoresis (2-DIGE) to analyze the brain tissue proteome, 76 differentially expressed proteins (p < 0.05; fold change>1.25) were identified between HAD and HIV non-dementia patients, of which 36 protein spots (based on 3D appearance of spots on the images) were chosen for the mass spectrometry analysis. The large majority of identified proteins were represented in the energy metabolic (mitochondria) and signal transduction pathways. Furthermore, over 90% of the protein candidates are common to both HAD and other non-viral neurodegenerative disease, such as Alzheimer's disease. The data was further validated using specific antibodies to 4 proteins (CA2, GS, CKMT and CRMP2) by western blot (WB) in the same samples used for 2D-DIGE, with additional confirmation by immunohistochemitsry (IHC) using frontal lobe tissue from different HAD and HIV+ non-dementia patients. The validation for all 4 antibodies by WB and IHC was in concordance with the DIGE results, lending further credence to the current findings.
Conclusion: These results suggest not only convergent pathogenetic pathways for the two diseases but also the possibility of increased Alzheimer's disease (AD) susceptibility in HAD patients whose life expectancy has been significantly increased by highly active antiretroviral therapy.
Figures



References
-
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. Journal of neurovirology. 2004;10:350–357. doi: 10.1080/13550280490521078. - DOI - PubMed
-
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS (London, England) 2003;17:1539–1545. - PubMed
-
- Sui Y, Potula R, Pinson D, Adany I, Li Z, Day J, Buch E, Segebrecht J, Villinger F, Liu Z, Huang M, Narayan O, Buch S. Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIV-encephalitis. Journal of medical primatology. 2003;32:229–239. doi: 10.1034/j.1600-0684.2003.00030.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

