Targeting surface nucleolin with a multivalent pseudopeptide delays development of spontaneous melanoma in RET transgenic mice
- PMID: 20573279
- PMCID: PMC2912263
- DOI: 10.1186/1471-2407-10-325
Targeting surface nucleolin with a multivalent pseudopeptide delays development of spontaneous melanoma in RET transgenic mice
Abstract
Background: The importance of cell-surface nucleolin in cancer biology was recently highlighted by studies showing that ligands of nucleolin play critical role in tumorigenesis and angiogenesis. By using a specific antagonist that binds the C-terminal tail of nucleolin, the HB-19 pseudopeptide, we recently reported that HB-19 treatment markedly suppressed the progression of established human breast tumor cell xenografts in the athymic nude mice without apparent toxicity.
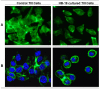
Methods: The in vivo antitumoral action of HB-19 treatment was assessed on the spontaneous development of melanoma in the RET transgenic mouse model. Ten days old RET mice were treated with HB-19 in a prophylactic setting that extended 300 days. In parallel, the molecular basis for the action of HB-19 was investigated on a melanoma cell line (called TIII) derived from a cutaneous nodule of a RET mouse.
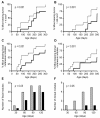
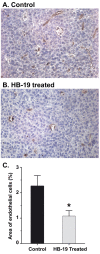
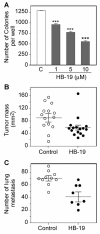
Results: HB-19 treatment of RET mice caused a significant delay in the onset of cutaneous tumors, several-months delay in the incidence of large tumors, a lower frequency of cutaneous nodules, and a reduction of visceral metastatic nodules while displaying no toxicity to normal tissue. Moreover, microvessel density was significantly reduced in tumors recovered from HB-19 treated mice compared to corresponding controls. Studies on the melanoma-derived tumor cells demonstrated that HB-19 treatment of TIII cells could restore contact inhibition, impair anchorage-independent growth, and reduce their tumorigenic potential in mice. Moreover, HB-19 treatment caused selective down regulation of transcripts coding matrix metalloproteinase 2 and 9, and tumor necrosis factor-alpha in the TIII cells and in melanoma tumors of RET mice.
Conclusions: Although HB-19 treatment failed to prevent the development of spontaneous melanoma in the RET mice, it delayed for several months the onset and frequency of cutaneous tumors, and exerted a significant inhibitory effect on visceral metastasis. Consequently, HB-19 could provide a novel therapeutic agent by itself or as an adjuvant therapy in association with current therapeutic interventions on a virulent cancer like melanoma.
Figures


Similar articles
-
Targeting surface nucleolin with multivalent HB-19 and related Nucant pseudopeptides results in distinct inhibitory mechanisms depending on the malignant tumor cell type.BMC Cancer. 2011 Aug 3;11:333. doi: 10.1186/1471-2407-11-333. BMC Cancer. 2011. PMID: 21812966 Free PMC article.
-
Suppression of tumorigenicity of rhabdoid tumor derived G401 cells by the multivalent HB-19 pseudopeptide that targets surface nucleolin.Biochimie. 2011 Mar;93(3):426-33. doi: 10.1016/j.biochi.2010.10.015. Epub 2010 Oct 30. Biochimie. 2011. PMID: 21040752
-
Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin.PLoS One. 2008 Jun 18;3(6):e2518. doi: 10.1371/journal.pone.0002518. PLoS One. 2008. PMID: 18560571 Free PMC article.
-
Interleukin 4-Induced Gene 1 as an Emerging Regulator of B-Cell Biology and its Role in Cutaneous Melanoma.Crit Rev Immunol. 2019;39(1):39-57. doi: 10.1615/CritRevImmunol.2019030020. Crit Rev Immunol. 2019. PMID: 31679193 Review.
-
Cell surface nucleolin as active bait for nanomedicine in cancer therapy: a promising option.Nanotechnology. 2021 May 17;32(32). doi: 10.1088/1361-6528/abfb30. Nanotechnology. 2021. PMID: 33892482 Review.
Cited by
-
Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex.Blood. 2013 Jun 6;121(23):4729-39. doi: 10.1182/blood-2012-12-471094. Epub 2013 Apr 18. Blood. 2013. PMID: 23599269 Free PMC article.
-
Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization.PLoS One. 2010 Dec 23;5(12):e15787. doi: 10.1371/journal.pone.0015787. PLoS One. 2010. PMID: 21203423 Free PMC article.
-
Multivalent cationic pseudopeptide polyplexes as a tool for cancer therapy.Oncotarget. 2017 Sep 30;8(52):90108-90122. doi: 10.18632/oncotarget.21441. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163814 Free PMC article.
-
Urokinase-type Plasminogen Activator (uPA) Promotes Angiogenesis by Attenuating Proline-rich Homeodomain Protein (PRH) Transcription Factor Activity and De-repressing Vascular Endothelial Growth Factor (VEGF) Receptor Expression.J Biol Chem. 2016 Jul 15;291(29):15029-45. doi: 10.1074/jbc.M115.678490. Epub 2016 May 4. J Biol Chem. 2016. PMID: 27151212 Free PMC article.
-
Nucleolin mediates the antiangiogenesis effect of the pseudopeptide N6L.BMC Cell Biol. 2012 Nov 13;13:32. doi: 10.1186/1471-2121-13-32. BMC Cell Biol. 2012. PMID: 23146273 Free PMC article.
References
-
- Srivastava M, Pollard HB. Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–1922. - PubMed
-
- Storck S, Shukla M, Dimitrov S, Bouvet P. Functions of the histone chaperone nucleolin in diseases. Subcell Biochem. 2007;41:125–44. full_text. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

