An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival
- PMID: 20573281
- PMCID: PMC2906432
- DOI: 10.1186/1471-2121-11-45
An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival
Abstract
Background: Sphingosine-1-phosphate (S1P) is a bioactive lipid that regulates a multitude of cellular functions, including cell proliferation, survival, migration and angiogenesis. S1P mediates its effects either by signaling through G protein-coupled receptors (GPCRs) or through an intracellular mode of action. In this study, we have investigated the mechanism behind S1P-induced survival signalling.
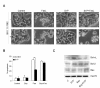
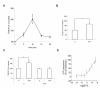
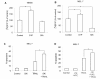
Results: We found that S1P protected cells from FasL-induced cell death in an NF-kappaB dependent manner. NF-kappaB was activated by extracellular S1P via S1P2 receptors and Gi protein signaling. Our study also demonstrates that extracellular S1P stimulates cells to rapidly produce and secrete additional S1P, which can further amplify the NF-kappaB activation.
Conclusions: We propose a self-amplifying loop of autocrine S1P with capacity to enhance cell survival. The mechanism provides increased understanding of the multifaceted roles of S1P in regulating cell fate during normal development and carcinogenesis.
Figures

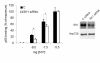


References
-
- Taha TA, Argraves KM, Obeid LM. Sphingosine phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. - PubMed
-
- Waters CM, Long J, Gorshkova I, Fujiwara Y, Connell M, Belmonte KE, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the Sphingosine 1-phosphate receptor-1. FASEB J. 2006;20:509–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

