A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards
- PMID: 20573448
- PMCID: PMC2921457
- DOI: 10.1016/j.pain.2010.05.028
A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards
Abstract
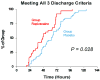
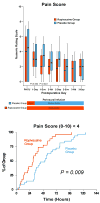
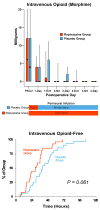
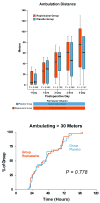
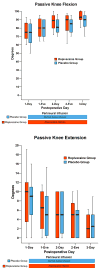
A continuous femoral nerve block (cFNB) involves the percutaneous insertion of a catheter adjacent to the femoral nerve, followed by a local anesthetic infusion, improving analgesia following total knee arthroplasty (TKA). Portable infusion pumps allow infusion continuation following hospital discharge, raising the possibility of decreasing hospitalization duration. We therefore used a multicenter, randomized, triple-masked, placebo-controlled study design to test the primary hypothesis that a 4-day ambulatory cFNB decreases the time until each of three predefined readiness-for-discharge criteria (adequate analgesia, independence from intravenous opioids, and ambulation 30m) are met following TKA compared with an overnight inpatient-only cFNB. Preoperatively, all patients received a cFNB with perineural ropivacaine 0.2% from surgery until the following morning, at which time they were randomized to either continue perineural ropivacaine (n=39) or switch to normal saline (n=38). Patients were discharged with their cFNB and portable infusion pump as early as postoperative day 3. Patients who were given 4 days of perineural ropivacaine attained all three criteria in a median (25th-75th percentiles) of 47 (29-69)h, compared with 62 (45-79)h for those of the control group (Estimated ratio=0.80, 95% confidence interval: 0.66-1.00; p=0.028). Compared with controls, patients randomized to ropivacaine met the discharge criterion for analgesia in 20 (0-38) versus 38 (15-64)h (p=0.009), and intravenous opioid independence in 21 (0-37) versus 33 (11-50)h (p=0.061). We conclude that a 4-day ambulatory cFNB decreases the time to reach three important discharge criteria by an estimated 20% following TKA compared with an overnight cFNB, primarily by improving analgesia.
Copyright (c) 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
References
-
- The Common Rule, Title 45 (Public Welfare), Code of Federal Regulations, Part 46 (Protection of Human Subjects) U.S. Department of Health and Human Services, National Institutes of Health, and Office for Human Research Protections; 2001. pp. 1–18. - PubMed
-
- Avidan A, Weissman C, Sprung CL. An internet web site as a data collection platform for multicenter research. Anesth Analg. 2005;100:506–11. - PubMed
-
- Bickler P, Brandes J, Lee M, Bozic K, Chesbro B, Claassen J. Bleeding complications from femoral and sciatic nerve catheters in patients receiving low molecular weight heparin. Anesth Analg. 2006;103:1036–1037. - PubMed
-
- Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–392. - PubMed
-
- Borgeat A, Kalberer F, Jacob H, Ruetsch YA, Gerber C. Patient-controlled interscalene analgesia with ropivacaine 0.2% versus bupivacaine 0.15% after major open shoulder surgery: The effects on hand motor function. Anesth Analg. 2001;92:218–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

