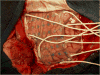
Subdural electrodes
- PMID: 20573543
- PMCID: PMC2962988
- DOI: 10.1016/j.clinph.2010.04.037
Subdural electrodes
Abstract
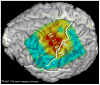
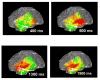
Subdural electrodes are frequently used to aid in the neurophysiological assessment of patients with intractable seizures. We review the indications for these, their uses for localizing epileptogenic regions and for localizing cortical regions supporting movement, sensation, and language.
2010 International Federation of Clinical Neurophysiology. Published by Elsevier Ireland Ltd. All rights reserved.
Figures




















References
-
- Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol. 1995;94:326–337. - PubMed
-
- Arroyo S, Lesser RP, Awad CA, Goldring S, Sutherling WW, Resnick TJ. Subdural and epidural grids and strips. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. Raven Press; New York: 1993. pp. 377–386.
-
- Arroyo S, Lesser RP, Gordon B, Jackson D. Mu rhythm in the human cortex: An electrophysiologic study with subdural electrodes. Neurology. 1992;42(Suppl 3):265. - PubMed
-
- Battaglia G, Chiapparini L, Franceschetti S, Freri E, Tassi L, Bassanini S, Villani F, Spreafico R, D'Incerti L, Granata T. Periventricular nodular heterotopia: classification, epileptic history, and genesis of epileptic discharges. Epilepsia. 2006;47:86–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

