A transient kinetic approach to investigate nucleoside inhibitors of mitochondrial DNA polymerase gamma
- PMID: 20573564
- PMCID: PMC2916041
- DOI: 10.1016/j.ymeth.2010.05.001
A transient kinetic approach to investigate nucleoside inhibitors of mitochondrial DNA polymerase gamma
Abstract
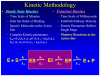
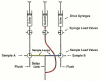
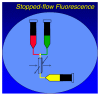
Nucleoside analogs play an essential role in treating human immunodeficiency virus (HIV) infection since the beginning of the AIDS epidemic and work by inhibition of HIV-1 reverse transcriptase (RT), a viral polymerase essential for DNA replication. Today, over 90% of all regimens for HIV treatment contain at least one nucleoside. Long-term use of nucleoside analogs has been associated with adverse effects including mitochondrial toxicity due to inhibition of the mitochondrial polymerase, DNA polymerase gamma (mtDNA pol gamma). In this review, we describe our efforts to delineate the molecular mechanism of nucleoside inhibition of HIV-1 RT and mtDNA pol gamma based upon a transient kinetic approach using rapid chemical quench methodology. Using transient kinetic methods, the maximum rate of polymerization (k(pol)), the dissociation constant for the ground state binding (K(d)), and the incorporation efficiency (k(pol)/K(d)) can be determined for the nucleoside analogs and their natural substrates. This analysis allowed us to develop an understanding of the structure activity relationships that allow correlation between the structural and stereochemical features of the nucleoside analog drugs with their mechanistic behavior toward the viral polymerase, RT, and the host cell polymerase, mtDNA pol gamma. An in-depth understanding of the mechanisms of inhibition of these enzymes is imperative in overcoming problems associated with toxicity.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

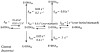
Similar articles
-
Perspectives on the molecular mechanism of inhibition and toxicity of nucleoside analogs that target HIV-1 reverse transcriptase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):296-9. doi: 10.1016/s0925-4439(02)00092-3. Biochim Biophys Acta. 2002. PMID: 12084471 Review.
-
Insights into the molecular mechanism of mitochondrial toxicity by AIDS drugs.J Biol Chem. 2001 Jun 29;276(26):23832-7. doi: 10.1074/jbc.M101156200. Epub 2001 Apr 27. J Biol Chem. 2001. PMID: 11328813
-
Balancing antiviral potency and host toxicity: identifying a nucleotide inhibitor with an optimal kinetic phenotype for HIV-1 reverse transcriptase.Mol Pharmacol. 2012 Jul;82(1):125-33. doi: 10.1124/mol.112.078758. Epub 2012 Apr 18. Mol Pharmacol. 2012. PMID: 22513406 Free PMC article.
-
An analysis of enzyme kinetics data for mitochondrial DNA strand termination by nucleoside reverse transcription inhibitors.PLoS Comput Biol. 2009 Jan;5(1):e1000261. doi: 10.1371/journal.pcbi.1000261. Epub 2009 Jan 9. PLoS Comput Biol. 2009. PMID: 19132079 Free PMC article.
-
Biochemical and mechanistic basis for the activity of nucleoside analogue inhibitors of HIV reverse transcriptase.Curr Top Med Chem. 2004;4(10):1035-44. doi: 10.2174/1568026043388358. Curr Top Med Chem. 2004. PMID: 15193137 Review.
Cited by
-
Sensitivity of mitochondrial transcription and resistance of RNA polymerase II dependent nuclear transcription to antiviral ribonucleosides.PLoS Pathog. 2012;8(11):e1003030. doi: 10.1371/journal.ppat.1003030. Epub 2012 Nov 15. PLoS Pathog. 2012. PMID: 23166498 Free PMC article.
-
Networked Communication between Polymerase and Exonuclease Active Sites in Human Mitochondrial DNA Polymerase.J Am Chem Soc. 2019 Jul 10;141(27):10821-10829. doi: 10.1021/jacs.9b04655. Epub 2019 Jun 28. J Am Chem Soc. 2019. PMID: 31251605 Free PMC article.
References
-
- De Clercq E. Anti-HIV drugs. Verh K Acad Geneeskd Belg. 2007;69(2):81–104. - PubMed
-
- Lewis W, Simpson JF, Meyer RR. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circulation.Research. 1994;74:344–348. - PubMed
-
- Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1(5):417–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

