Signaling from the secretory granule to the nucleus: Uhmk1 and PAM
- PMID: 20573687
- PMCID: PMC2940467
- DOI: 10.1210/me.2009-0381
Signaling from the secretory granule to the nucleus: Uhmk1 and PAM
Abstract
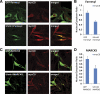
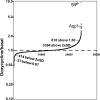
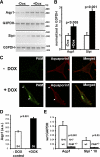
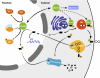
Neurons and endocrine cells package peptides in secretory granules (large dense-core vesicles) for storage and stimulated release. Studies of peptidylglycine alpha-amidating monooxygenase (PAM), an essential secretory granule membrane enzyme, revealed a pathway that can relay information from secretory granules to the nucleus, resulting in alterations in gene expression. The cytosolic domain (CD) of PAM, a type 1 membrane enzyme essential for the production of amidated peptides, is basally phosphorylated by U2AF homology motif kinase 1 (Uhmk1) and other Ser/Thr kinases. Proopiomelanocortin processing in AtT-20 corticotrope tumor cells was increased when Uhmk1 expression was reduced. Uhmk1 was concentrated in the nucleus, but cycled rapidly between nucleus and cytosol. Endoproteolytic cleavage of PAM releases a soluble CD fragment that localizes to the nucleus. Localization of PAM-CD to the nucleus was decreased when PAM-CD with phosphomimetic mutations was examined and when active Uhmk1 was simultaneously overexpressed. Membrane-tethering Uhmk1 did not eliminate its ability to exclude PAM-CD from the nucleus, suggesting that cytosolic Uhmk1 could cause this response. Microarray analysis demonstrated the ability of PAM to increase expression of a small subset of genes, including aquaporin 1 (Aqp1) in AtT-20 cells. Aqp1 mRNA levels were higher in wild-type mice than in mice heterozygous for PAM, indicating that a similar relationship occurs in vivo. Expression of PAM-CD also increased Aqp1 levels whereas expression of Uhmk1 diminished Aqp1 expression. The outlines of a pathway that ties secretory granule metabolism to the transcriptome are thus apparent.
Figures


Similar articles
-
A histidine-rich linker region in peptidylglycine α-amidating monooxygenase has the properties of a pH sensor.J Biol Chem. 2014 May 2;289(18):12404-20. doi: 10.1074/jbc.M113.545947. Epub 2014 Mar 13. J Biol Chem. 2014. PMID: 24627494 Free PMC article.
-
Secretion stimulates intramembrane proteolysis of a secretory granule membrane enzyme.J Biol Chem. 2010 Nov 5;285(45):34632-42. doi: 10.1074/jbc.M110.145334. Epub 2010 Sep 3. J Biol Chem. 2010. PMID: 20817724 Free PMC article.
-
Access of a membrane protein to secretory granules is facilitated by phosphorylation.J Biol Chem. 2001 Oct 26;276(43):40326-37. doi: 10.1074/jbc.M011460200. Epub 2001 Aug 27. J Biol Chem. 2001. PMID: 11524414
-
Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains.Protein Sci. 1993 Apr;2(4):489-97. doi: 10.1002/pro.5560020401. Protein Sci. 1993. PMID: 8518727 Free PMC article. Review.
-
60 YEARS OF POMC: From POMC and α-MSH to PAM, molecular oxygen, copper, and vitamin C.J Mol Endocrinol. 2016 May;56(4):T63-76. doi: 10.1530/JME-15-0266. Epub 2015 Dec 14. J Mol Endocrinol. 2016. PMID: 26667899 Free PMC article. Review.
Cited by
-
Probing the production of amidated peptides following genetic and dietary copper manipulations.PLoS One. 2011;6(12):e28679. doi: 10.1371/journal.pone.0028679. Epub 2011 Dec 16. PLoS One. 2011. PMID: 22194882 Free PMC article.
-
Pam heterozygous mice reveal essential role for Cu in amygdalar behavioral and synaptic function.Ann N Y Acad Sci. 2014 May;1314(1):15-23. doi: 10.1111/nyas.12378. Epub 2014 Mar 4. Ann N Y Acad Sci. 2014. PMID: 24593825 Free PMC article.
-
Signaling from the secretory granule to the nucleus.Crit Rev Biochem Mol Biol. 2012 Jul-Aug;47(4):391-406. doi: 10.3109/10409238.2012.694845. Epub 2012 Jun 8. Crit Rev Biochem Mol Biol. 2012. PMID: 22681236 Free PMC article. Review.
-
UHMK1 promotes gastric cancer progression through reprogramming nucleotide metabolism.EMBO J. 2020 Mar 2;39(5):e102541. doi: 10.15252/embj.2019102541. Epub 2020 Jan 23. EMBO J. 2020. PMID: 31975428 Free PMC article.
-
A pH-sensitive luminal His-cluster promotes interaction of PAM with V-ATPase along the secretory and endocytic pathways of peptidergic cells.J Cell Physiol. 2019 Jun;234(6):8683-8697. doi: 10.1002/jcp.27528. Epub 2018 Oct 14. J Cell Physiol. 2019. PMID: 30317586 Free PMC article.
References
-
- Ferraro F, Eipper BA, Mains RE 2005 Retrieval and reuse of pituitary secretory granule proteins. J Biol Chem 280:25424–25435 - PubMed
-
- Vo YP, Hutton JC, Angleson JK 2004 Recycling of the dense-core vesicle membrane protein phogrin in Min6 β-cells. Biochem Biophys Res Commun 324:1004–1010 - PubMed
-
- Milgram SL, Mains RE, Eipper BA 1996 Identification of routing determinants in the cytosolic domain of a secretory granule-associated integral membrane protein. J Biol Chem 271:17526–17535 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

