Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium
- PMID: 20573694
- PMCID: PMC2927697
- DOI: 10.1242/dev.049718
Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium
Abstract
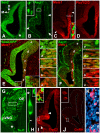
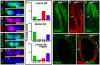
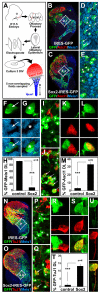
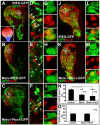
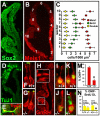
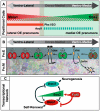
Neural precursors in the developing olfactory epithelium (OE) give rise to three major neuronal classes - olfactory receptor (ORNs), vomeronasal (VRNs) and gonadotropin releasing hormone (GnRH) neurons. Nevertheless, the molecular and proliferative identities of these precursors are largely unknown. We characterized two precursor classes in the olfactory epithelium (OE) shortly after it becomes a distinct tissue at midgestation in the mouse: slowly dividing self-renewing precursors that express Meis1/2 at high levels, and rapidly dividing neurogenic precursors that express high levels of Sox2 and Ascl1. Precursors expressing high levels of Meis genes primarily reside in the lateral OE, whereas precursors expressing high levels of Sox2 and Ascl1 primarily reside in the medial OE. Fgf8 maintains these expression signatures and proliferative identities. Using electroporation in the wild-type embryonic OE in vitro as well as Fgf8, Sox2 and Ascl1 mutant mice in vivo, we found that Sox2 dose and Meis1 - independent of Pbx co-factors - regulate Ascl1 expression and the transition from lateral to medial precursor state. Thus, we have identified proliferative characteristics and a dose-dependent transcriptional network that define distinct OE precursors: medial precursors that are most probably transit amplifying neurogenic progenitors for ORNs, VRNs and GnRH neurons, and lateral precursors that include multi-potent self-renewing OE neural stem cells.
Figures


Similar articles
-
Identity, lineage and fates of a temporally distinct progenitor population in the embryonic olfactory epithelium.Dev Biol. 2023 Mar;495:76-91. doi: 10.1016/j.ydbio.2023.01.001. Epub 2023 Jan 7. Dev Biol. 2023. PMID: 36627077 Free PMC article.
-
A novel embryonic nestin-expressing radial glia-like progenitor gives rise to zonally restricted olfactory and vomeronasal neurons.J Neurosci. 2008 Apr 16;28(16):4271-82. doi: 10.1523/JNEUROSCI.5566-07.2008. J Neurosci. 2008. PMID: 18417707 Free PMC article.
-
Specific mesenchymal/epithelial induction of olfactory receptor, vomeronasal, and gonadotropin-releasing hormone (GnRH) neurons.Dev Dyn. 2010 Jun;239(6):1723-38. doi: 10.1002/dvdy.22315. Dev Dyn. 2010. PMID: 20503368 Free PMC article.
-
Feedback Regulation of Neurogenesis in the Mammalian Olfactory Epithelium: New Insights from Genetics and Systems Biology.In: Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 10. In: Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 10. PMID: 21882434 Free Books & Documents. Review.
-
Identification and molecular regulation of neural stem cells in the olfactory epithelium.Exp Cell Res. 2005 Jun 10;306(2):309-16. doi: 10.1016/j.yexcr.2005.03.027. Epub 2005 Apr 21. Exp Cell Res. 2005. PMID: 15925585 Review.
Cited by
-
A Balanced Translocation in Kallmann Syndrome Implicates a Long Noncoding RNA, RMST, as a GnRH Neuronal Regulator.J Clin Endocrinol Metab. 2020 Mar 1;105(3):e231-44. doi: 10.1210/clinem/dgz011. J Clin Endocrinol Metab. 2020. PMID: 31628846 Free PMC article.
-
Progressive effects of N-myc deficiency on proliferation, neurogenesis, and morphogenesis in the olfactory epithelium.Dev Neurobiol. 2014 Jun;74(6):643-56. doi: 10.1002/dneu.22162. Epub 2014 Jan 16. Dev Neurobiol. 2014. PMID: 24376126 Free PMC article.
-
Foxd4 is essential for establishing neural cell fate and for neuronal differentiation.Genesis. 2017 Jun;55(6):10.1002/dvg.23031. doi: 10.1002/dvg.23031. Epub 2017 Apr 3. Genesis. 2017. PMID: 28316121 Free PMC article.
-
Specification of GnRH-1 neurons by antagonistic FGF and retinoic acid signaling.Dev Biol. 2012 Feb 15;362(2):254-62. doi: 10.1016/j.ydbio.2011.12.016. Epub 2011 Dec 19. Dev Biol. 2012. PMID: 22200593 Free PMC article.
-
sox2 and sox3 Play unique roles in development of hair cells and neurons in the zebrafish inner ear.Dev Biol. 2018 Mar 1;435(1):73-83. doi: 10.1016/j.ydbio.2018.01.010. Epub 2018 Jan 31. Dev Biol. 2018. PMID: 29355523 Free PMC article.
References
-
- Ache B. W., Young J. M. (2005). Olfaction: diverse species, conserved principles. Neuron 48, 417-430 - PubMed
-
- Anchan R. M., Drake D. P., Haines C. F., Gerwe E. A., LaMantia A. S. (1997). Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. J. Comp. Neurol. 379, 171-184 - PubMed
-
- Asson-Batres M. A., Smith W. B. (2006). Localization of retinaldehyde dehydrogenases and retinoid binding proteins to sustentacular cells, glia, Bowman's gland cells, and stroma: potential sites of retinoic acid synthesis in the postnatal rat olfactory organ. J. Comp. Neurol. 496, 149-171 - PMC - PubMed
-
- Bani-Yaghoub M., Tremblay R. G., Lei J. X., Zhang D., Zurakowski B., Sandhu J. K., Smith B., Ribecco-Lutkiewicz M., Kennedy J., Walker P. R., et al. (2006). Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 295, 52-66 - PubMed
-
- Beites C. L., Kawauchi S., Crocker C. E., Calof A. L. (2005). Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp. Cell Res. 306, 309-316 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

