Self-avoidance and tiling: Mechanisms of dendrite and axon spacing
- PMID: 20573716
- PMCID: PMC2926746
- DOI: 10.1101/cshperspect.a001750
Self-avoidance and tiling: Mechanisms of dendrite and axon spacing
Abstract
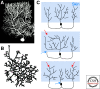
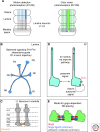
The spatial pattern of branches within axonal or dendritic arbors and the relative arrangement of neighboring arbors with respect to one another impact a neuron's potential connectivity. Although arbors can adopt diverse branching patterns to suit their functions, evenly spread branches that avoid clumping or overlap are a common feature of many axonal and dendritic arbors. The degree of overlap between neighboring arbors innervating a surface is also characteristic within particular neuron types. The arbors of some populations of neurons innervate a target with a comprehensive and nonoverlapping "tiled" arrangement, whereas those of others show substantial territory overlap. This review focuses on cellular and molecular studies that have provided insight into the regulation of spatial arrangements of neurite branches within and between arbors. These studies have revealed principles that govern arbor arrangements in dendrites and axons in both vertebrates and invertebrates. Diverse molecular mechanisms controlling the spatial patterning of sister branches and neighboring arbors have begun to be elucidated.
Figures


Similar articles
-
[Molecular basis for establishment and maintenance of dendritic trees].Brain Nerve. 2008 Apr;60(4):351-64. Brain Nerve. 2008. PMID: 18421977 Review. Japanese.
-
Morphological Diversity Strongly Constrains Synaptic Connectivity and Plasticity.Cereb Cortex. 2017 Sep 1;27(9):4570-4585. doi: 10.1093/cercor/bhx150. Cereb Cortex. 2017. PMID: 28637203
-
A universal property of axonal and dendritic arbors.Neuron. 2010 Apr 15;66(1):45-56. doi: 10.1016/j.neuron.2010.02.013. Neuron. 2010. PMID: 20399728
-
Maximization of the connectivity repertoire as a statistical principle governing the shapes of dendritic arbors.Proc Natl Acad Sci U S A. 2009 Jul 28;106(30):12536-41. doi: 10.1073/pnas.0901530106. Epub 2009 Jul 21. Proc Natl Acad Sci U S A. 2009. PMID: 19622738 Free PMC article.
-
Development of dendritic form and function.Annu Rev Cell Dev Biol. 2015;31:741-77. doi: 10.1146/annurev-cellbio-100913-013020. Epub 2015 Sep 29. Annu Rev Cell Dev Biol. 2015. PMID: 26422333 Review.
Cited by
-
Selective disruption of trigeminal sensory neurogenesis and differentiation in a mouse model of 22q11.2 deletion syndrome.Dis Model Mech. 2022 Feb 1;15(2):dmm047357. doi: 10.1242/dmm.047357. Epub 2021 May 4. Dis Model Mech. 2022. PMID: 33722956 Free PMC article.
-
The role of cell adhesion molecules in visual circuit formation: from neurite outgrowth to maps and synaptic specificity.Dev Neurobiol. 2015 Jun;75(6):569-83. doi: 10.1002/dneu.22267. Epub 2015 Feb 19. Dev Neurobiol. 2015. PMID: 25649254 Free PMC article. Review.
-
Convergent local identity and topographic projection of sensory neurons.J Neurosci. 2011 Nov 23;31(47):17017-27. doi: 10.1523/JNEUROSCI.2815-11.2011. J Neurosci. 2011. PMID: 22114271 Free PMC article.
-
Self-referential forces are sufficient to explain different dendritic morphologies.Front Neuroinform. 2013 Jan 30;7:1. doi: 10.3389/fninf.2013.00001. eCollection 2013. Front Neuroinform. 2013. PMID: 23386828 Free PMC article.
-
The seven-pass transmembrane cadherin Flamingo controls dendritic self-avoidance via its binding to a LIM domain protein, Espinas, in Drosophila sensory neurons.Genes Dev. 2011 Sep 15;25(18):1982-96. doi: 10.1101/gad.16531611. Genes Dev. 2011. PMID: 21937715 Free PMC article.
References
-
- Ashley JA, Katz FN 1994. Competition and position-dependent targeting in the development of the Drosophila R7 visual projections. Development 120: 1537–1547 - PubMed
-
- Baker MW, Macagno ER 2000. The role of a LAR-like receptor tyrosine phosphatase in growth cone collapse and mutual-avoidance by sibling processes. J Neurobiol 44: 194–203 - PubMed
-
- Baker MW, Macagno ER 2007. In vivo imaging of growth cone and filopodial dynamics: Evidence for contact-mediated retraction of filopodia leading to the tiling of sibling processes. J Comp Neurol 500: 850–862 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
