Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility
- PMID: 20573723
- PMCID: PMC5398471
- DOI: 10.1210/en.2010-0314
Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility
Abstract
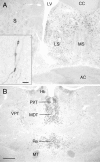
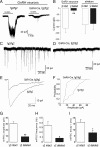
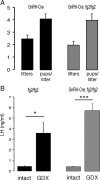
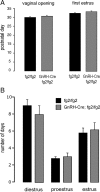
The amino acid gamma-aminobutyric acid (GABA) is thought to play a key role in shaping the activity of the GnRH neurons throughout embryonic and postnatal life. However, the physiological roles of direct GABA inputs to GnRH neurons remain unknown. Using a Cre-LoxP strategy, we generated a targeted mouse line, in which all (98 +/- 1%) GnRH neurons had the gamma2-subunit of the GABA(A) receptor deleted. Electrophysiological recordings of GABA(A)-mediated postsynaptic currents from green fluorescent protein-tagged GnRH neurons with the gamma2-subunit knocked out (GnRH gamma2 KO) showed that the amplitude and frequency of GABA(A) postsynaptic currents were reduced by 70% (P < 0.01) and 77% (P < 0.05), respectively, and that the response to exogenous GABA was reduced by 90% (P < 0.01). Evaluation of male and female GnRH gamma2 KO mice revealed completely normal fecundity, estrous cycles, and puberty onset. Further investigation with gonadectomy and different steroid replacement regimens showed normal basal levels of LH in both sexes, and a normal estradiol-evoked positive feedback mechanism in females. However, the increment in LH after gonadectomy in GnRH gamma2 KO female mice was double that of controls (P < 0.05) and also more potently suppressed by 17-beta-estradiol (P < 0.05). A similar but nonsignificant trend was observed in GnRH gamma2 KO male mice. Together, these findings show that 70-90% reductions in the normal levels of GABA(A) receptor activity at the GnRH neuron appear to impact upon the estrogen negative feedback mechanism but are, nevertheless, compatible with normal fertility in mice.
Figures
Similar articles
-
The role of cAMP response element-binding protein in estrogen negative feedback control of gonadotropin-releasing hormone neurons.J Neurosci. 2012 Aug 15;32(33):11309-17. doi: 10.1523/JNEUROSCI.1333-12.2012. J Neurosci. 2012. PMID: 22895714 Free PMC article.
-
Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability.J Neurosci. 2011 Feb 16;31(7):2421-30. doi: 10.1523/JNEUROSCI.5759-10.2011. J Neurosci. 2011. PMID: 21325509 Free PMC article.
-
Expression of ESR1 in Glutamatergic and GABAergic Neurons Is Essential for Normal Puberty Onset, Estrogen Feedback, and Fertility in Female Mice.J Neurosci. 2015 Oct 28;35(43):14533-43. doi: 10.1523/JNEUROSCI.1776-15.2015. J Neurosci. 2015. PMID: 26511244 Free PMC article.
-
Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics.Cell Calcium. 2012 Mar-Apr;51(3-4):267-76. doi: 10.1016/j.ceca.2011.11.005. Epub 2011 Dec 15. Cell Calcium. 2012. PMID: 22177387 Review.
-
Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus.J Neuroendocrinol. 2011 Jul;23(7):557-69. doi: 10.1111/j.1365-2826.2011.02145.x. J Neuroendocrinol. 2011. PMID: 21518033 Free PMC article. Review.
Cited by
-
Genetic factors in precocious puberty.Clin Exp Pediatr. 2022 Apr;65(4):172-181. doi: 10.3345/cep.2021.00521. Epub 2021 Oct 18. Clin Exp Pediatr. 2022. PMID: 34665958 Free PMC article.
-
Modulation of Gonadotropin-Releasing Hormone Neuron Activity and Secretion in Mice by Non-peptide Neurotransmitters, Gasotransmitters, and Gliotransmitters.Front Endocrinol (Lausanne). 2019 May 22;10:329. doi: 10.3389/fendo.2019.00329. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31178828 Free PMC article. Review.
-
Characterization and Comparative Analysis of Whole-Transcriptome Sequencing in High- and Low-Fecundity Chongming White Goat Ovaries during the Estrus Phase.Animals (Basel). 2024 Mar 22;14(7):988. doi: 10.3390/ani14070988. Animals (Basel). 2024. PMID: 38612227 Free PMC article.
-
The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type.Front Neural Circuits. 2014 Feb 3;8:3. doi: 10.3389/fncir.2014.00003. eCollection 2014. Front Neural Circuits. 2014. PMID: 24550784 Free PMC article. Review.
-
Chemogenetic Depletion of Hypophysiotropic GnRH Neurons Does Not Affect Fertility in Mature Female Zebrafish.Int J Mol Sci. 2022 May 17;23(10):5596. doi: 10.3390/ijms23105596. Int J Mol Sci. 2022. PMID: 35628411 Free PMC article.
References
-
- Han SK, Todman MG, Herbison AE 2004. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145:495–499 - PubMed
-
- Moenter SM, DeFazio RA 2005. Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 - PubMed
-
- Sim JA, Skynner MJ, Pape JR, Herbison AE 2000. Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci 12:3497–3504 - PubMed
-
- Pape JR, Skynner MJ, Sim JA, Herbison AE 2001. Profiling γ-aminobutyric acid (GABA(A)) receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology 74:300–308 - PubMed
-
- Jung H, Shannon EM, Fritschy JM, Ojeda SR 1998. Several GABAA receptor subunits are expressed in LHRH neurons of juvenile female rats. Brain Research 780:218–229 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

