Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus
- PMID: 20573725
- PMCID: PMC2940495
- DOI: 10.1210/en.2010-0223
Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus
Abstract
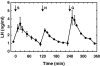
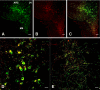
Human genetics indicate that kisspeptin and neurokinin B (NKB) signaling are necessary for generating pulsatile LH release and therefore for initiation of puberty and maintaining gonadal function. In the present study, male monkeys were employed to examine 1) whether activation of the NKB receptor (NK3R) is associated with GnRH release, and 2) hypothalamic localization of these peptides using immunofluorescence histochemistry. Agonadal juveniles, in which pituitary responsiveness to GnRH was heightened by GnRH priming, were employed to indirectly examine GnRH-releasing actions of NK3R and kisspeptin receptor agonists by tracking LH after their i.v. injection. Castrated adults were used for immunohistochemistry. Single i.v. injections of NKB or senktide (an NK3R agonist) elicited robust LH discharges that were abolished by GnRH receptor antagonism (acyline) confirming the ligands' hypothalamic action. Intermittent infusion of senktide (1-min pulse every hour for 4 h), in contrast to that of kisspeptin, failed to sustain pulsatile GnRH release. Repetitive senktide injections did not compromise the GnRH-releasing action of kisspeptin. NKB and kisspeptin were colocalized in perikarya of the arcuate nucleus and in axonal projections to the median eminence, confirming earlier findings in sheep. These results are consistent with the human genetics, and indicate that although brief activation of NK3R stimulates GnRH release, repetitive stimulation of this pathway, in contrast to that of kisspeptin receptor, fails to sustain pulsatile GnRH release. In addition, the data provide a platform for future elucidation of the interactions between NKB and kisspeptin that are required for generating pulsatile GnRH release in primates.
Figures






Comment in
-
Neurokinin B and kisspeptin: sexual partners or single agents?Endocrinology. 2010 Sep;151(9):4090-1. doi: 10.1210/en.2010-0696. Endocrinology. 2010. PMID: 20736403 No abstract available.
References
-
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Apaicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 - PubMed
-
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2008 New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 - PubMed

