Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects
- PMID: 20573787
- PMCID: PMC2921536
- DOI: 10.3945/ajcn.2010.29642
Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects
Abstract
Background: The intake of selenium needed for optimal health has not been established. Selenoproteins perform the functions of selenium, and the selenium intake needed for their full expression is not known.
Objective: This study sought to determine the intake of selenium required to optimize plasma selenoprotein P (SEPP1) and to compare SEPP1 with other plasma selenium biomarkers.
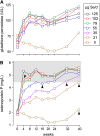
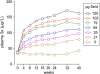
Design: A 40-wk placebo-controlled, double-blind study of selenium repletion was carried out in 98 healthy Chinese subjects who had a daily dietary selenium intake of 14 micro g. Fourteen subjects each were assigned randomly to daily dose groups of 0, 21, 35, 55, 79, 102, and 125 micro g Se as l-selenomethionine. Plasma glutathione peroxidase (GPX) activity, SEPP1, and selenium were measured. A biomarker was considered to be optimized when its value was not different from the mean value of the subjects receiving larger supplements.
Results: The SEPP1 concentration was optimized at 40 wk by the 35- micro g supplement, which indicated that 49 micro g/d could optimize it. GPX activity was optimized by 21 micro g (total ingestion: 35 micro g/d). The selenium concentration showed no tendency to become optimized.
Conclusions: The present results indicate that SEPP1 concentration is the best plasma biomarker studied for assessing optimal expression of all selenoproteins, because its optimization required a larger intake of selenium than did GPX activity. On the basis of the selenium intake needed for SEPP1 optimization with adjustments for body weight and individual variation, ap 75 micro g Se/d as selenomethionine is postulated to allow full expression of selenoproteins in US residents. This trial was registered at clinicaltrials.gov as NCT00428649.
Figures
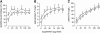
Similar articles
-
Selenium deficiency occurs in some patients with moderate-to-severe cirrhosis and can be corrected by administration of selenate but not selenomethionine: a randomized controlled trial.Am J Clin Nutr. 2015 Nov;102(5):1126-33. doi: 10.3945/ajcn.115.110932. Epub 2015 Oct 14. Am J Clin Nutr. 2015. PMID: 26468123 Free PMC article. Clinical Trial.
-
Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial.Am J Clin Nutr. 2010 Apr;91(4):923-31. doi: 10.3945/ajcn.2009.28169. Epub 2010 Feb 24. Am J Clin Nutr. 2010. PMID: 20181815 Free PMC article. Clinical Trial.
-
Calculation of an Adequate Intake (AI) Value and Safe Range of Selenium (Se) for Chinese Infants 0-3 Months Old Based on Se Concentration in the Milk of Lactating Chinese Women with Optimal Se Intake.Biol Trace Elem Res. 2019 Apr;188(2):363-372. doi: 10.1007/s12011-018-1440-9. Epub 2018 Jul 16. Biol Trace Elem Res. 2019. PMID: 30014285
-
Regulation of Selenium Metabolism and Transport.Annu Rev Nutr. 2015;35:109-34. doi: 10.1146/annurev-nutr-071714-034250. Epub 2015 May 13. Annu Rev Nutr. 2015. PMID: 25974694 Review.
-
Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism.Free Radic Biol Med. 2013 Dec;65:1538-1547. doi: 10.1016/j.freeradbiomed.2013.07.016. Epub 2013 Jul 18. Free Radic Biol Med. 2013. PMID: 23872396 Review.
Cited by
-
Decreased Concentration of Fibroblast Growth Factor 23 (FGF-23) as a Result of Supplementation with Selenium and Coenzyme Q10 in an Elderly Swedish Population: A Sub-Analysis.Cells. 2022 Feb 1;11(3):509. doi: 10.3390/cells11030509. Cells. 2022. PMID: 35159318 Free PMC article. Clinical Trial.
-
Selenium, Zinc, and Copper Status of Vegetarians and Vegans in Comparison to Omnivores in the Nutritional Evaluation (NuEva) Study.Nutrients. 2023 Aug 11;15(16):3538. doi: 10.3390/nu15163538. Nutrients. 2023. PMID: 37630729 Free PMC article.
-
Decrease in inflammatory biomarker concentration by intervention with selenium and coenzyme Q10: a subanalysis of osteopontin, osteoprotergerin, TNFr1, TNFr2 and TWEAK.J Inflamm (Lond). 2019 Mar 18;16:5. doi: 10.1186/s12950-019-0210-6. eCollection 2019. J Inflamm (Lond). 2019. PMID: 30923464 Free PMC article.
-
The Possible Mechanism of Physiological Adaptation to the Low-Se Diet and Its Health Risk in the Traditional Endemic Areas of Keshan Diseases.Biol Trace Elem Res. 2022 May;200(5):2069-2083. doi: 10.1007/s12011-021-02851-7. Epub 2021 Aug 8. Biol Trace Elem Res. 2022. PMID: 34365573 Free PMC article. Review.
-
Linear association of compound dietary antioxidant index with hyperlipidemia: a cross-sectional study.Front Nutr. 2024 Feb 29;11:1365580. doi: 10.3389/fnut.2024.1365580. eCollection 2024. Front Nutr. 2024. PMID: 38487634 Free PMC article.
References
-
- Xia Y, Piao J-H, Hill KE, Burk RF. Keshan disease and selenium status of populations in China. : Burk RF, Selenium in biology and human health. New York, NY: Springer Verlag, 1994:182–96
-
- Xia Y. Keshan disease. : Kiple KF, Ornelas KC, The Cambridge world history of food. New York, NY: Cambridge University Press, 2000:939–47
-
- Yang GQ.Keshan Disease: an endemic selenium-related deficiency disease. : Chandra RK, Trace elements in nutrition of children. New York, NY: Vevey/Raven Press, 1985:273–90
-
- Sutter ME, Thomas JD, Brown J, Morgan B. Selenium toxicity: a case of selenosis caused by a nutritional supplement. Ann Intern Med 2008;148:970–1 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

