Alpha interferon and not gamma interferon inhibits salmonid alphavirus subtype 3 replication in vitro
- PMID: 20573808
- PMCID: PMC2919011
- DOI: 10.1128/JVI.00851-10
Alpha interferon and not gamma interferon inhibits salmonid alphavirus subtype 3 replication in vitro
Abstract
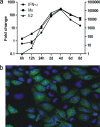
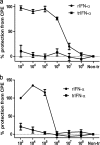
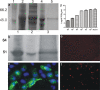
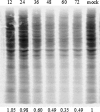
Salmonid alphavirus (SAV) is an emerging virus in salmonid aquaculture, with SAV-3 being the only subtype found in Norway. Until now, there has been little focus on the alpha interferon (IFN-alpha)-induced antiviral responses during virus infection in vivo or in vitro in fish. The possible involvement of IFN-gamma in the response to SAV-3 is also not known. In this study, the two IFNs were cloned and expressed as recombinant proteins (recombinant IFN-alpha [rIFN-alpha] and rIFN-gamma) and used for in vitro studies. SAV-3 infection in a permissive salmon cell line (TO cells) results in IFN-alpha and IFN-stimulated gene (ISG) mRNA upregulation. Preinfection treatment (4 to 24 h prior to infection) with salmon rIFN-alpha induces an antiviral state that inhibits the replication of SAV-3 and protects the cells against virus-induced cytopathic effects (CPE). The antiviral state coincides with a strong expression of Mx and ISG15 mRNA and Mx protein expression. When rIFN-alpha is administered at the time of infection and up to 24 h postinfection, virus replication is not inhibited, and cells are not protected against virus-induced CPE. By 40 h postinfection, the alpha subunit of eukaryotic initiation factor 2 (eIF2alpha) is phosphorylated concomitant with the expression of the E2 protein as assessed by Western blotting. Postinfection treatment with rIFN-alpha results in a moderate reduction in E2 expression levels in accordance with a moderate downregulation of cellular protein synthesis, an approximately 65% reduction by 60 h postinfection. rIFN-gamma has only a minor inhibitory effect on SAV-3 replication in vitro. SAV-3 is sensitive to the preinfection antiviral state induced by rIFN-alpha, while postinfection antiviral responses or postinfection treatment with rIFN-alpha is not able to limit viral replication.
Figures





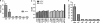
Similar articles
-
Expression of interferon and interferon--induced genes in Atlantic salmon Salmo salar cell lines SHK-1 and TO following infection with Salmon AlphaVirus SAV.Fish Shellfish Immunol. 2009 Apr;26(4):672-5. doi: 10.1016/j.fsi.2009.02.021. Epub 2009 Mar 3. Fish Shellfish Immunol. 2009. PMID: 19264132
-
Atlantic salmon kidney (ASK) cells are an effective model to characterise interferon (IFN) and IFN-induced gene expression following salmonid alphavirus infection.Fish Shellfish Immunol. 2020 Nov;106:792-795. doi: 10.1016/j.fsi.2020.08.043. Epub 2020 Aug 29. Fish Shellfish Immunol. 2020. PMID: 32871248
-
The C-Terminal Domain of Salmonid Alphavirus Nonstructural Protein 2 (nsP2) Is Essential and Sufficient To Block RIG-I Pathway Induction and Interferon-Mediated Antiviral Response.J Virol. 2021 Nov 9;95(23):e0115521. doi: 10.1128/JVI.01155-21. Epub 2021 Sep 15. J Virol. 2021. PMID: 34523969 Free PMC article.
-
Alphavirus infections in salmonids--a review.J Fish Dis. 2007 Sep;30(9):511-31. doi: 10.1111/j.1365-2761.2007.00848.x. J Fish Dis. 2007. PMID: 17718707 Review.
-
Expression of interferon and interferon-induced genes in salmonids in response to virus infection, interferon-inducing compounds and vaccination.Fish Shellfish Immunol. 2008 Oct;25(4):351-7. doi: 10.1016/j.fsi.2008.02.004. Epub 2008 Feb 10. Fish Shellfish Immunol. 2008. PMID: 18400516 Review.
Cited by
-
Development of infectious cDNA clones of Salmonid alphavirus subtype 3.BMC Res Notes. 2010 Sep 21;3:241. doi: 10.1186/1756-0500-3-241. BMC Res Notes. 2010. PMID: 20858233 Free PMC article.
-
Experimental piscine alphavirus RNA recombination in vivo yields both viable virus and defective viral RNA.Sci Rep. 2016 Nov 2;6:36317. doi: 10.1038/srep36317. Sci Rep. 2016. PMID: 27805034 Free PMC article.
-
Stress-induced reversion to virulence of infectious pancreatic necrosis virus in naïve fry of Atlantic salmon (Salmo salar L.).PLoS One. 2013;8(2):e54656. doi: 10.1371/journal.pone.0054656. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431359 Free PMC article.
-
Identification of genetic loci associated with higher resistance to pancreas disease (PD) in Atlantic salmon (Salmo salar L.).BMC Genomics. 2020 Jun 3;21(1):388. doi: 10.1186/s12864-020-06788-4. BMC Genomics. 2020. PMID: 32493246 Free PMC article.
-
PKR Activation Favors Infectious Pancreatic Necrosis Virus Replication in Infected Cells.Viruses. 2016 Jun 21;8(6):173. doi: 10.3390/v8060173. Viruses. 2016. PMID: 27338445 Free PMC article.
References
-
- Berg, T. S. K., B. Sun, and B. Robertsen. 2009. An antiserum against Atlantic salmon IFNa1 detects IFN and neutralizes antiviral activity produced by poly I:C stimulated cells. Dev. Comp. Immunol. 33:638-645. - PubMed
-
- Bergan, V., R. Jagus, S. Lauksund, O. Kileng, and B. Robertsen. 2008. The Atlantic salmon Z-DNA binding protein kinase phosphorylates translation initiation factor 2 alpha and constitutes a unique orthologue to the mammalian dsRNA-activated protein kinase R. FEBS J. 275:184-197. - PubMed
-
- Brehin, A. C., I. Casademont, M. P. Frenkiel, C. Julier, A. Sakuntabhai, and P. Despres. 2009. The large form of human 2′,5′-oligoadenylate synthetase (OAS3) exerts antiviral effect against Chikungunya virus. Virology 384:216-222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

