Distinct molecular pathways to X4 tropism for a V3-truncated human immunodeficiency virus type 1 lead to differential coreceptor interactions and sensitivity to a CXCR4 antagonist
- PMID: 20573813
- PMCID: PMC2919036
- DOI: 10.1128/JVI.00333-10
Distinct molecular pathways to X4 tropism for a V3-truncated human immunodeficiency virus type 1 lead to differential coreceptor interactions and sensitivity to a CXCR4 antagonist
Abstract
During the course of infection, transmitted HIV-1 isolates that initially use CCR5 can acquire the ability to use CXCR4, which is associated with an accelerated progression to AIDS. Although this coreceptor switch is often associated with mutations in the stem of the viral envelope (Env) V3 loop, domains outside V3 can also play a role, and the underlying mechanisms and structural basis for how X4 tropism is acquired remain unknown. In this study we used a V3 truncated R5-tropic Env as a starting point to derive two X4-tropic Envs, termed DeltaV3-X4A.c5 and DeltaV3-X4B.c7, which took distinct molecular pathways for this change. The DeltaV3-X4A.c5 Env clone acquired a 7-amino-acid insertion in V3 that included three positively charged residues, reestablishing an interaction with the CXCR4 extracellular loops (ECLs) and rendering it highly susceptible to the CXCR4 antagonist AMD3100. In contrast, the DeltaV3-X4B.c7 Env maintained the V3 truncation but acquired mutations outside V3 that were critical for X4 tropism. In contrast to DeltaV3-X4A.c5, DeltaV3-X4B.c7 showed increased dependence on the CXCR4 N terminus (NT) and was completely resistant to AMD3100. These results indicate that HIV-1 X4 coreceptor switching can involve (i) V3 loop mutations that establish interactions with the CXCR4 ECLs, and/or (ii) mutations outside V3 that enhance interactions with the CXCR4 NT. The cooperative contributions of CXCR4 NT and ECL interactions with gp120 in acquiring X4 tropism likely impart flexibility on pathways for viral evolution and suggest novel approaches to isolate these interactions for drug discovery.
Figures

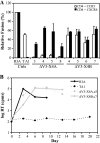



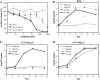
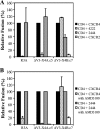
References
-
- Agrawal-Gamse, C., F. H. Lee, B. Haggarty, A. P. Jordan, Y. Yi, B. Lee, R. G. Collman, J. A. Hoxie, R. W. Doms, and M. M. Laakso. 2009. Adaptive mutations in a human immunodeficiency virus type 1 envelope protein with a truncated V3 loop restore function by improving interactions with CD4. J. Virol. 83:11005-11015. - PMC - PubMed
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Atchison, R. E., J. Gosling, F. S. Monteclaro, C. Franci, L. Digilio, I. F. Charo, and M. A. Goldsmith. 1996. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science 274:1924-1926. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

