Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B
- PMID: 20573816
- PMCID: PMC2919031
- DOI: 10.1128/JVI.00169-10
Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B
Abstract
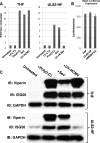
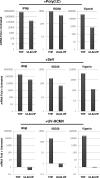
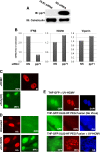
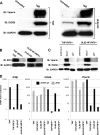
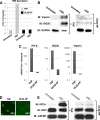
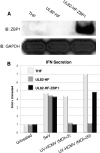
In vitro infection of cells with the betaherpesvirus human cytomegalovirus (HCMV) stimulates an innate immune response characterized by phosphorylation of the transcription factor interferon regulatory factor 3 (IRF3) and subsequent expression of IRF3-dependent genes. While previous work suggests that HCMV envelope glycoprotein B is responsible for initiating this reaction, the signaling pathways stimulated by virus infection that lead to IRF3 phosphorylation have largely been uncharacterized. Recently, we identified Z DNA binding protein 1 (ZBP1), a sensor of cytoplasmic DNA, as an essential protein for this response. We now describe a human fibroblast cell line exhibiting a recessive defect that results in the absence of activation of IRF3 following treatment with HCMV but not Sendai virus or double-stranded RNA. In addition, we show that while exposure of these cells to soluble HCMV glycoprotein B is capable of triggering IRF3-dependent gene transcription, transfection of the cells with double-stranded DNA is not. Furthermore, we show that overexpression of ZBP1 in these cells reestablishes their ability to secrete interferon in response to HCMV and that multiple ZBP1 transcriptional variants exist in both wild-type and mutant cells. These results have two major implications for the understanding of innate immune stimulation by HCMV. First, they demonstrate that HCMV glycoprotein B is not the essential molecular pattern that induces an IRF3-dependent innate immune response. Second, IRF3-terminal signaling triggered by HCMV particles closely resembles that which is activated by cytoplasmic double-stranded DNA.
Figures


References
-
- Alford, C. A., S. Stagno, R. F. Pass, and W. J. Britt. 1990. Congenital and perinatal cutomegalovirus infection. Rev. Infect. Dis. 12:745-753. - PubMed
-
- Ankel, H., D. F. Westra, S. Welling-Wester, and P. Lebon. 1998. Induction of interferon-alpha by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology 251:317-326. - PubMed
-
- Boudinot, P., S. Riffault, S. Salhi, C. Carrat, C. Sedlik, N. Mahmoudi, B. Charley, and A. Benmansour. 2000. Vesicular stomatitis virus and pseudorabies virus induce a vig1/cig5 homologue in mouse dendritic cells via different pathways. J. Gen. Virol. 81:2675-2682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

