Delaying the expression of herpes simplex virus type 1 glycoprotein B (gB) to a true late gene alters neurovirulence and inhibits the gB-CD8+ T-cell response in the trigeminal ganglion
- PMID: 20573821
- PMCID: PMC2919033
- DOI: 10.1128/JVI.00496-10
Delaying the expression of herpes simplex virus type 1 glycoprotein B (gB) to a true late gene alters neurovirulence and inhibits the gB-CD8+ T-cell response in the trigeminal ganglion
Abstract
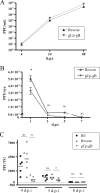
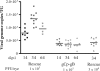
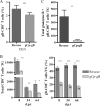
Following herpes simplex virus type 1 (HSV-1) ocular infection of C57BL/6 mice, activated CD8(+) T cells specific for an immunodominant epitope on HSV-1 glycoprotein B (gB-CD8 cells) establish a stable memory population in HSV-1 latently infected trigeminal ganglia (TG), whereas non-HSV-specific CD8(+) T cells are lost over time. The retention and activation of gB-CD8 cells appear to be influenced by persistent viral antigenic exposure within the latently infected TG. We hypothesized that the low-level expression of gB from its native promoter before viral DNA synthesis is critical for the retention and activation of gB-CD8 cells in the TG during HSV-1 latency and for their ability to block HSV-1 reactivation from latency. To test this, we created a recombinant HSV-1 in which gB is expressed only after viral DNA synthesis from the true late gC promoter (gCp-gB). Despite minor growth differences compared to its rescuant in infected corneas, gCp-gB was significantly growth impaired in the TG and produced a reduced latent genome load. The gCp-gB- and rescuant-infected mice mounted similar gB-CD8 effector responses, but the size and activation phenotypes of the memory gB-CD8 cells were diminished in gCp-gB latently infected TG, suggesting that the stimulation of gB-CD8 cells requires gB expression before viral DNA synthesis. Surprisingly, late gB expression did not compromise the capacity of gB-CD8 cells to inhibit HSV-1 reactivation from latency in ex vivo TG cultures, suggesting that gB-CD8 cells can block HSV-1 reactivation at a very late stage in the viral life cycle. These data have implications for designing better immunogens for vaccines to prevent HSV-1 reactivation.
Figures


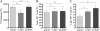
Similar articles
-
Herpes Simplex Virus 1-Specific CD8+ T Cell Priming and Latent Ganglionic Retention Are Shaped by Viral Epitope Promoter Kinetics.J Virol. 2020 Feb 14;94(5):e01193-19. doi: 10.1128/JVI.01193-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826989 Free PMC article.
-
Influence of an immunodominant herpes simplex virus type 1 CD8+ T cell epitope on the target hierarchy and function of subdominant CD8+ T cells.PLoS Pathog. 2017 Dec 4;13(12):e1006732. doi: 10.1371/journal.ppat.1006732. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29206240 Free PMC article.
-
Dok-1 and Dok-2 Are Required To Maintain Herpes Simplex Virus 1-Specific CD8+ T Cells in a Murine Model of Ocular Infection.J Virol. 2017 Jul 12;91(15):e02297-16. doi: 10.1128/JVI.02297-16. Print 2017 Aug 1. J Virol. 2017. PMID: 28490594 Free PMC article.
-
CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation.J Neurovirol. 2011 Dec;17(6):528-34. doi: 10.1007/s13365-011-0062-1. Epub 2011 Dec 8. J Neurovirol. 2011. PMID: 22161682 Review.
-
Control of HSV-1 latency in human trigeminal ganglia--current overview.J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
-
UL11 Protein Is a Key Participant of the Duck Plague Virus in Its Life Cycle.Front Microbiol. 2022 Jan 4;12:792361. doi: 10.3389/fmicb.2021.792361. eCollection 2021. Front Microbiol. 2022. PMID: 35058907 Free PMC article.
-
Expression of immunogenic structural proteins of cyprinid herpesvirus 3 in vitro assessed using immunofluorescence.Vet Res. 2016 Jan 8;47:8. doi: 10.1186/s13567-015-0297-6. Vet Res. 2016. PMID: 26742989 Free PMC article.
-
Herpes simplex virus and varicella zoster virus, the house guests who never leave.Herpesviridae. 2012 Jun 12;3(1):5. doi: 10.1186/2042-4280-3-5. Herpesviridae. 2012. PMID: 22691604 Free PMC article.
-
Herpes Simplex Virus 1-Specific CD8+ T Cell Priming and Latent Ganglionic Retention Are Shaped by Viral Epitope Promoter Kinetics.J Virol. 2020 Feb 14;94(5):e01193-19. doi: 10.1128/JVI.01193-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826989 Free PMC article.
-
A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections.J Virol. 2012 Jun;86(12):6563-74. doi: 10.1128/JVI.00302-12. Epub 2012 Apr 4. J Virol. 2012. PMID: 22491465 Free PMC article.
References
-
- Bedoui, S., P. G. Whitney, J. Waithman, L. Eidsmo, L. Wakim, I. Caminschi, R. S. Allan, M. Wojtasiak, K. Shortman, F. R. Carbone, A. G. Brooks, and W. R. Heath. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488-495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

