Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test and identification of rare polymorphisms potentially affecting assay performance
- PMID: 20573864
- PMCID: PMC2916612
- DOI: 10.1128/JCM.00776-10
Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test and identification of rare polymorphisms potentially affecting assay performance
Abstract
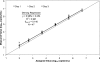
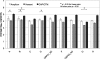
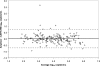
We evaluated the FDA-approved Roche Cobas AmpliPrep/Cobas TaqMan (CAP/CTM) HIV-1 viral load assay for sensitivity, reproducibility, linearity, HIV-1 subtype detection, and correlation to the Roche Amplicor HIV-1 monitor test, version 1.5 (Amplicor). The limit of detection calculated by probit analysis was 23.8 copies/ml using the 2nd International WHO Standard and 30.8 copies/ml using Viral Quality Assurance (VQA) standard material. Serial dilutions of six patient samples were used to determine inter- and intra-assay reproducibility and linearity, which were very good (<8% coefficient of variation [CV]; between approximately 1.7 and 7.0 log(10) copies/ml). Subtype detection was evaluated in the CAP/CTM, Amplicor, and Bayer Versant HIV-1 bDNA 3.0 (Versant) assays using a commercially available panel. Versant averaged 0.829 log(10) copies/ml lower than CAP/CTM and Amplicor averaged 0.427 log(10) copies/ml lower than CAP/CTM for the subtype panel. Correlation with samples previously tested by Amplicor was excellent (R(2) = 0.884; average difference [Amplicor value minus CAP/CTM value], 0.008 log(10) copies/ml). Of the 305 HIV samples tested, 7 samples generated CAP/CTM titers between 1.0 and 2.75 log(10) copies/ml lower than those for Amplicor. Three of these samples revealed primer and probe mismatches that could account for the discrepancies. Otherwise, the CAP/CTM assay exhibits excellent sensitivity, dynamic range, reproducibility, and correlation with Amplicor in an automated format.
Figures
Similar articles
-
Performance evaluation of the new Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test version 2.0 for quantification of human immunodeficiency virus type 1 RNA.J Clin Microbiol. 2010 Apr;48(4):1195-200. doi: 10.1128/JCM.01832-09. Epub 2010 Feb 17. J Clin Microbiol. 2010. PMID: 20164281 Free PMC article.
-
Correction of underquantification of human immunodeficiency virus type 1 load with the second version of the Roche Cobas AmpliPrep/Cobas TaqMan assay.J Clin Microbiol. 2010 Apr;48(4):1337-42. doi: 10.1128/JCM.01226-09. Epub 2010 Feb 17. J Clin Microbiol. 2010. PMID: 20164284 Free PMC article.
-
Comparative evaluation of the Cobas Amplicor HIV-1 Monitor Ultrasensitive Test, the new Cobas AmpliPrep/Cobas Amplicor HIV-1 Monitor Ultrasensitive Test and the Versant HIV RNA 3.0 assays for quantitation of HIV-1 RNA in plasma samples.J Clin Virol. 2005 May;33(1):43-51. doi: 10.1016/j.jcv.2004.09.025. J Clin Virol. 2005. PMID: 15797364
-
Evaluation of the Cobas AmpliPrep/Cobas Amplicor HIV-1 Monitor Ultrasensitive Test: comparison with the Cobas Amplicor HIV-1 Monitor test (manual specimen preparation).J Clin Virol. 2002 Dec;25 Suppl 3:S103-7. doi: 10.1016/s1386-6532(02)00185-3. J Clin Virol. 2002. PMID: 12467784
-
Performance of NucliSens HIV-1 EasyQ Version 2.0 compared with six commercially available quantitative nucleic acid assays for detection of HIV-1 in China.Mol Diagn Ther. 2010 Oct 1;14(5):305-16. doi: 10.1007/BF03256386. Mol Diagn Ther. 2010. PMID: 21053996
Cited by
-
The impact of dolutegravir-based combination antiretroviral therapy on the spermatozoa and fertility parameters of men living with human immunodeficiency virus.Andrologia. 2022 Dec;54(11):e14621. doi: 10.1111/and.14621. Epub 2022 Oct 19. Andrologia. 2022. PMID: 36261884 Free PMC article.
-
The scanning electron microscope in microbiology and diagnosis of infectious disease.Sci Rep. 2016 May 23;6:26516. doi: 10.1038/srep26516. Sci Rep. 2016. PMID: 27212232 Free PMC article.
-
Characterizing Patients with Very-Low-Level HIV Viremia: A Community-Based Study.J Int Assoc Provid AIDS Care. 2017 May/Jun;16(3):261-266. doi: 10.1177/2325957416680028. Epub 2016 Nov 30. J Int Assoc Provid AIDS Care. 2017. PMID: 27903948 Free PMC article.
-
Large-scale analysis of the prevalence and geographic distribution of HIV-1 non-B variants in the United States.J Clin Microbiol. 2013 Aug;51(8):2662-9. doi: 10.1128/JCM.00880-13. Epub 2013 Jun 12. J Clin Microbiol. 2013. PMID: 23761148 Free PMC article.
-
Cost savings associated with testing of antibodies, antigens, and nucleic acids for diagnosis of acute HIV infection.J Clin Microbiol. 2012 Jun;50(6):1874-8. doi: 10.1128/JCM.00106-12. Epub 2012 Mar 21. J Clin Microbiol. 2012. PMID: 22442319 Free PMC article.
References
-
- Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. - PubMed
-
- Braun, P., R. Ehret, F. Wiesmann, F. Zabbai, M. Knickmann, R. Kühn, S. Thamm, G. Warnat, and H. Knechten. 2007. Comparison of four commercial quantitative HIV-1 assays for viral load monitoring in clinical daily routine. Clin. Chem. Lab. Med. 45:93-99. - PubMed
-
- Damond, F., B. Roquebert, A. Bénard, G. Collin, M. Miceli, P. Yéni, F. Brun-Vezinet, and D. Descamps. 2007. Human immunodeficiency virus type 1 (HIV-1) plasma load discrepancies between the Roche COBAS AMPLICOR HIV-1 MONITOR version 1.5 and the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 assays. J. Clin. Microbiol. 45:3436-3438. - PMC - PubMed
-
- Delaugerre, C., B. Denis, G. Peytavin, P. Palmer, T. Mourez, J. Le Goff, J. Molina, and F. Simon. 2009. Clinical and resistance consequences of misquantification of plasma and cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) RNA in an HIV-1 subtype G-infected patient. J. Clin. Microbiol. 47:3763-3764. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

