Aplysia cell adhesion molecule and a novel protein kinase C activity in the postsynaptic neuron are required for presynaptic growth and initial formation of specific synapses
- PMID: 20573882
- PMCID: PMC2908027
- DOI: 10.1523/JNEUROSCI.0546-10.2010
Aplysia cell adhesion molecule and a novel protein kinase C activity in the postsynaptic neuron are required for presynaptic growth and initial formation of specific synapses
Abstract
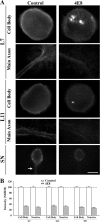
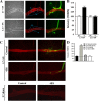
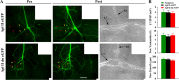
To explore the role of both Aplysia cell adhesion molecule (ApCAM) and activity of specific protein kinase C (PKC) isoforms in the initial formation of sensory neuron synapses with specific postsynaptic targets (L7 but not L11), we examined presynaptic growth, initial synapse formation, and the expression of the presynaptic neuropeptide sensorin following cell-specific reduction of ApCAM or of a novel PKC activity. Synapse formation between sensory neurons and L7 begins by 3 h after plating and is accompanied by a rapid accumulation of a novel PKC to sites of synaptic interaction. Reducing ApCAM expression specifically from the surface of L7 blocks presynaptic growth and initial synapse formation, target-induced increase of sensorin in sensory neuron cell bodies and the rapid accumulation of the novel PKC to sites of interaction. Selective blockade of the novel PKC activity in L7, but not in sensory neurons, with injection of a dominant negative construct that interferes with the novel PKC activity, produces the same actions as downregulating ApCAM; blockade of presynaptic growth and initial synapse formation, and the target-induced increase of sensorin in sensory neuron cell bodies. The results indicate that signals initiated by postsynaptic cell adhesion molecule ApCAM coupled with the activation of a novel PKC in the appropriate postsynaptic neuron produce the retrograde signals required for presynaptic growth associated with initial synapse formation, and the target-induced expression of a presynaptic neuropeptide critical for synapse maturation.
Figures






Similar articles
-
Multifunctional role of protein kinase C in regulating the formation and maturation of specific synapses.J Neurosci. 2007 Oct 24;27(43):11712-24. doi: 10.1523/JNEUROSCI.3305-07.2007. J Neurosci. 2007. PMID: 17959813 Free PMC article.
-
Changes in expression and distribution of Aplysia cell adhesion molecules can influence synapse formation and elimination in vitro.J Neurosci. 1995 Jun;15(6):4173-83. doi: 10.1523/JNEUROSCI.15-06-04173.1995. J Neurosci. 1995. PMID: 7790903 Free PMC article.
-
Target-dependent release of a presynaptic neuropeptide regulates the formation and maturation of specific synapses in Aplysia.J Neurosci. 2004 Nov 3;24(44):9933-43. doi: 10.1523/JNEUROSCI.3329-04.2004. J Neurosci. 2004. PMID: 15525778 Free PMC article.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
Silent synapses sit and wait for a better day.Neuron. 2009 Jan 29;61(2):157-9. doi: 10.1016/j.neuron.2009.01.004. Neuron. 2009. PMID: 19186159 Free PMC article. Review.
Cited by
-
Cell-Specific PKM Isoforms Contribute to the Maintenance of Different Forms of Persistent Long-Term Synaptic Plasticity.J Neurosci. 2017 Mar 8;37(10):2746-2763. doi: 10.1523/JNEUROSCI.2805-16.2017. Epub 2017 Feb 8. J Neurosci. 2017. PMID: 28179558 Free PMC article.
-
cJun and CREB2 in the postsynaptic neuron contribute to persistent long-term facilitation at a behaviorally relevant synapse.J Neurosci. 2015 Jan 7;35(1):386-95. doi: 10.1523/JNEUROSCI.3284-14.2015. J Neurosci. 2015. PMID: 25568130 Free PMC article.
-
Persistent Associative Plasticity at an Identified Synapse Underlying Classical Conditioning Becomes Labile with Short-Term Homosynaptic Activation.J Neurosci. 2015 Dec 9;35(49):16159-70. doi: 10.1523/JNEUROSCI.2034-15.2015. J Neurosci. 2015. PMID: 26658867 Free PMC article.
-
Persistent long-term synaptic plasticity requires activation of a new signaling pathway by additional stimuli.J Neurosci. 2011 Jun 15;31(24):8841-50. doi: 10.1523/JNEUROSCI.1358-11.2011. J Neurosci. 2011. PMID: 21677168 Free PMC article.
-
Possible novel features of synaptic regulation during long-term facilitation in Aplysia.Learn Mem. 2021 Jun 15;28(7):218-227. doi: 10.1101/lm.053124.120. Print 2021 Jul. Learn Mem. 2021. PMID: 34131053 Free PMC article.
References
-
- Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of ApCAM: an early step of learning-related synaptic growth in Aplysia . Science. 1992;256:645–649. - PubMed
-
- Bailey CH, Kaang BK, Chen M, Martin KC, Lim CS, Casadio A, Kandel ER. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of ApCAM in Aplysia sensory neurons. Neuron. 1997;18:913–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
