Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels
- PMID: 20573883
- PMCID: PMC6634643
- DOI: 10.1523/JNEUROSCI.4164-08.2010
Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels
Abstract
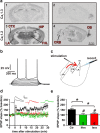
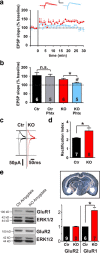
Ca(2+) influx through postsynaptic Ca(v)1.x L-type voltage-gated channels (LTCCs) is particularly effective in activating neuronal biochemical signaling pathways that might be involved in Hebbian synaptic plasticity (i.e., long-term potentiation and depression) and learning and memory. Here, we demonstrate that Ca(v)1.2 is the functionally relevant LTCC isoform in the thalamus-amygdala pathway of mice. We further show that acute pharmacological block of LTCCs abolishes Hebbian plasticity in the thalamus-amygdala pathway and impairs the acquisition of conditioned fear. On the other hand, chronic genetic loss of Ca(v)1.2 triggers a homeostatic change of the synapse, leading to a fundamental alteration of the mechanism of Hebbian plasticity by synaptic incorporation of Ca(2+)-permeable, GluA2-lacking AMPA receptors. Our results demonstrate for the first time the importance of the Ca(v)1.2 LTCC subtype in synaptic plasticity and fear memory acquisition.
Figures



Similar articles
-
Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala.Nature. 1998 Aug 13;394(6694):683-7. doi: 10.1038/29312. Nature. 1998. PMID: 9716132
-
Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits.Mol Neurobiol. 1996 Aug;13(1):1-22. doi: 10.1007/BF02740749. Mol Neurobiol. 1996. PMID: 8892333 Review.
-
Forebrain NR2B overexpression enhancing fear acquisition and long-term potentiation in the lateral amygdala.Eur J Neurosci. 2015 Sep;42(5):2214-23. doi: 10.1111/ejn.13008. Epub 2015 Jul 25. Eur J Neurosci. 2015. PMID: 26118841
-
Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways.Neuron. 2006 Dec 7;52(5):883-96. doi: 10.1016/j.neuron.2006.10.010. Neuron. 2006. PMID: 17145508 Free PMC article.
-
Interplay of amygdala and cingulate plasticity in emotional fear.Neural Plast. 2011;2011:813749. doi: 10.1155/2011/813749. Epub 2011 Sep 7. Neural Plast. 2011. PMID: 21912749 Free PMC article. Review.
Cited by
-
Late-Onset Behavioral and Synaptic Consequences of L-Type Ca2+ Channel Activation in the Basolateral Amygdala of Developing Rats.eNeuro. 2022 Feb 22;9(1):ENEURO.0282-21.2022. doi: 10.1523/ENEURO.0282-21.2022. Print 2022 Jan-Feb. eNeuro. 2022. PMID: 35064022 Free PMC article.
-
Calcineurin/P-ERK/Egr-1 Pathway is Involved in Fear Memory Impairment after Isoflurane Exposure in Mice.Sci Rep. 2017 Oct 24;7(1):13947. doi: 10.1038/s41598-017-13975-z. Sci Rep. 2017. PMID: 29066839 Free PMC article.
-
Calcium channel blocking as a therapeutic strategy for Alzheimer's disease: the case for isradipine.Biochim Biophys Acta. 2011 Dec;1812(12):1584-90. doi: 10.1016/j.bbadis.2011.08.013. Epub 2011 Sep 8. Biochim Biophys Acta. 2011. PMID: 21925266 Free PMC article. Review.
-
Cacna1c Hemizygosity Results in Aberrant Fear Conditioning to Neutral Stimuli.Schizophr Bull. 2020 Sep 21;46(5):1231-1238. doi: 10.1093/schbul/sbz127. Schizophr Bull. 2020. PMID: 31910256 Free PMC article.
-
Neuronal deletion of CaV1.2 is associated with sex-specific behavioral phenotypes in mice.Sci Rep. 2022 Dec 22;12(1):22152. doi: 10.1038/s41598-022-26504-4. Sci Rep. 2022. PMID: 36550186 Free PMC article.
References
-
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
