Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel beta subunits via paradoxical effects on persistent sodium currents
- PMID: 20573896
- PMCID: PMC6634624
- DOI: 10.1523/JNEUROSCI.1534-10.2010
Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel beta subunits via paradoxical effects on persistent sodium currents
Abstract
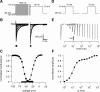
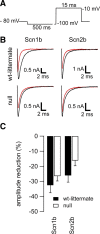
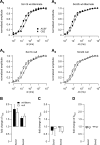
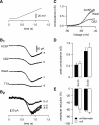
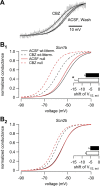
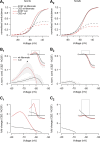
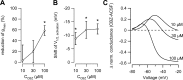
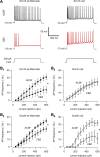
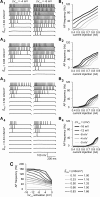
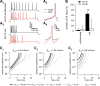
Neuronal excitability is critically determined by the properties of voltage-gated Na(+) currents. Fast transient Na(+) currents (I(NaT)) mediate the fast upstroke of action potentials, whereas low-voltage-activated persistent Na(+) currents (I(NaP)) contribute to subthreshold excitation. Na(+) channels are composed of a pore-forming alpha subunit and beta subunits, which modify the biophysical properties of alpha subunits. We have examined the idea that the presence of beta subunits also modifies the pharmacological properties of the Na(+) channel complex using mice lacking either the beta(1) (Scn1b) or beta(2) (Scn2b) subunit. Classical effects of the anticonvulsant carbamazepine (CBZ), such as the use-dependent reduction of I(NaT) and effects on I(NaT) voltage dependence of inactivation, were unaltered in mice lacking beta subunits. Surprisingly, CBZ induced a small but significant shift of the voltage dependence of activation of I(NaT) and I(NaP) to more hyperpolarized potentials. This novel CBZ effect on I(NaP) was strongly enhanced in Scn1b null mice, leading to a pronounced increase of I(NaP) within the subthreshold potential range, in particular at low CBZ concentrations of 10-30 microm. A combination of current-clamp and computational modeling studies revealed that this effect causes a complete loss of CBZ efficacy in reducing repetitive firing. Thus, beta subunits modify not only the biophysical but also the pharmacological properties of Na(+) channels, in particular with respect to I(NaP). Consequently, altered expression of beta subunits in other neurological disorders may cause altered neuronal sensitivity to drugs targeting Na(+) channels.
Figures

Similar articles
-
Loss of β1 accessory Na+ channel subunits causes failure of carbamazepine, but not of lacosamide, in blocking high-frequency firing via differential effects on persistent Na+ currents.Epilepsia. 2012 Nov;53(11):1959-67. doi: 10.1111/j.1528-1167.2012.03675.x. Epub 2012 Sep 27. Epilepsia. 2012. PMID: 23016711
-
The effects of eslicarbazepine on persistent Na⁺ current and the role of the Na⁺ channel β subunits.Epilepsy Res. 2014 Feb;108(2):202-11. doi: 10.1016/j.eplepsyres.2013.11.022. Epub 2013 Dec 8. Epilepsy Res. 2014. PMID: 24368131
-
Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels.J Neurosci. 2009 Feb 18;29(7):2027-42. doi: 10.1523/JNEUROSCI.4531-08.2009. J Neurosci. 2009. PMID: 19228957 Free PMC article.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
-
Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy.Neurochem Res. 2017 Sep;42(9):2495-2504. doi: 10.1007/s11064-017-2314-9. Epub 2017 Jun 7. Neurochem Res. 2017. PMID: 28589518 Free PMC article. Review.
Cited by
-
The cellular building blocks of breathing.Compr Physiol. 2012 Oct;2(4):2683-731. doi: 10.1002/cphy.c110033. Compr Physiol. 2012. PMID: 23720262 Free PMC article. Review.
-
PharmGKB summary: carbamazepine pathway.Pharmacogenet Genomics. 2011 Dec;21(12):906-10. doi: 10.1097/FPC.0b013e328348c6f2. Pharmacogenet Genomics. 2011. PMID: 21738081 Free PMC article. No abstract available.
-
A thermosensitive mutation alters the effects of lacosamide on slow inactivation in neuronal voltage-gated sodium channels, NaV1.2.Front Pharmacol. 2013 Sep 20;4:121. doi: 10.3389/fphar.2013.00121. eCollection 2013. Front Pharmacol. 2013. PMID: 24065921 Free PMC article.
-
Actions of the antiseizure drug carbamazepine in the thalamic reticular nucleus: Potential mechanism of aggravating absence seizures.Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2500644122. doi: 10.1073/pnas.2500644122. Epub 2025 Jul 31. Proc Natl Acad Sci U S A. 2025. PMID: 40743388
-
Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease.Neurosci Lett. 2010 Dec 10;486(2):53-9. doi: 10.1016/j.neulet.2010.06.050. Epub 2010 Jun 23. Neurosci Lett. 2010. PMID: 20600605 Free PMC article. Review.
References
-
- Catterall WA. Molecular properties of brain sodium channels: an important target for anticonvulsant drugs. Adv Neurol. 1999;79:441–456. - PubMed
-
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, 3rd, Kazen-Gillespie KA, Kazarinova-Noyes K, Shrager P, Saunders TL, Macdonald RL, Ransom BR, Scheuer T, Catterall WA, Isom LL. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–17077. - PMC - PubMed
-
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O'Malley HA, Bharucha V, Meadows LS, Knudsen GA, Vilaythong A, Noebels JL, Saunders TL, Scheuer T, Shrager P, Catterall WA, Isom LL. Mice lacking sodium channel β1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
