Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito
- PMID: 20573956
- PMCID: PMC2930704
- DOI: 10.1074/jbc.M110.122275
Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito
Abstract
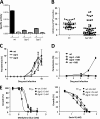
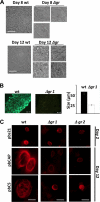
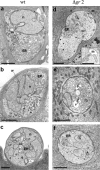
Malaria parasites contain a complete glutathione (GSH) redox system, and several enzymes of this system are considered potential targets for antimalarial drugs. Through generation of a gamma-glutamylcysteine synthetase (gamma-GCS)-null mutant of the rodent parasite Plasmodium berghei, we previously showed that de novo GSH synthesis is not critical for blood stage multiplication but is essential for oocyst development. In this study, phenotype analyses of mutant parasites lacking expression of glutathione reductase (GR) confirmed that GSH metabolism is critical for the mosquito oocyst stage. Similar to what was found for gamma-GCS, GR is not essential for blood stage growth. GR-null parasites showed the same sensitivity to methylene blue and eosin B as wild type parasites, demonstrating that these compounds target molecules other than GR in Plasmodium. Attempts to generate parasites lacking both GR and gamma-GCS by simultaneous disruption of gr and gamma-gcs were unsuccessful. This demonstrates that the maintenance of total GSH levels required for blood stage survival is dependent on either de novo GSH synthesis or glutathione disulfide (GSSG) reduction by Plasmodium GR. Our studies provide new insights into the role of the GSH system in malaria parasites with implications for the development of drugs targeting GSH metabolism.
Figures


Similar articles
-
The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission.PLoS Pathog. 2009 Feb;5(2):e1000302. doi: 10.1371/journal.ppat.1000302. Epub 2009 Feb 20. PLoS Pathog. 2009. PMID: 19229315 Free PMC article.
-
Glutathione-deficient Plasmodium berghei parasites exhibit growth delay and nuclear DNA damage.Free Radic Biol Med. 2016 Jun;95:43-54. doi: 10.1016/j.freeradbiomed.2016.02.032. Epub 2016 Mar 4. Free Radic Biol Med. 2016. PMID: 26952808 Free PMC article.
-
Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase.J Biol Chem. 2011 Sep 16;286(37):32661-71. doi: 10.1074/jbc.M111.269399. Epub 2011 Jul 19. J Biol Chem. 2011. PMID: 21771793 Free PMC article.
-
A novel genetic technique in Plasmodium berghei allows liver stage analysis of genes required for mosquito stage development and demonstrates that de novo heme synthesis is essential for liver stage development in the malaria parasite.PLoS Pathog. 2017 Jun 15;13(6):e1006396. doi: 10.1371/journal.ppat.1006396. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28617870 Free PMC article.
-
Biologic and pharmacologic regulation of mammalian glutathione synthesis.Free Radic Biol Med. 1999 Nov;27(9-10):922-35. doi: 10.1016/s0891-5849(99)00176-8. Free Radic Biol Med. 1999. PMID: 10569625 Review.
Cited by
-
Real-time imaging of the intracellular glutathione redox potential in the malaria parasite Plasmodium falciparum.PLoS Pathog. 2013;9(12):e1003782. doi: 10.1371/journal.ppat.1003782. Epub 2013 Dec 5. PLoS Pathog. 2013. PMID: 24348249 Free PMC article.
-
Cytostatic versus cytocidal profiling of quinoline drug combinations via modified fixed-ratio isobologram analysis.Malar J. 2013 Sep 18;12:332. doi: 10.1186/1475-2875-12-332. Malar J. 2013. PMID: 24044530 Free PMC article.
-
Implications of Glutathione Levels in the Plasmodium berghei Response to Chloroquine and Artemisinin.PLoS One. 2015 May 26;10(5):e0128212. doi: 10.1371/journal.pone.0128212. eCollection 2015. PLoS One. 2015. PMID: 26010448 Free PMC article.
-
Rapid and Specific Action of Methylene Blue against Plasmodium Transmission Stages.Pharmaceutics. 2022 Dec 14;14(12):2794. doi: 10.3390/pharmaceutics14122794. Pharmaceutics. 2022. PMID: 36559287 Free PMC article.
-
Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue.Proc Natl Acad Sci U S A. 2011 Nov 22;108(47):E1214-23. doi: 10.1073/pnas.1112037108. Epub 2011 Oct 31. Proc Natl Acad Sci U S A. 2011. PMID: 22042867 Free PMC article.
References
-
- Becker K., Tilley L., Vennerstrom J. L., Roberts D., Rogerson S., Ginsburg H. (2004) Int. J. Parasitol. 34, 163–189 - PubMed
-
- Becker K., Rahlfs S., Nickel C., Schirmer R. H. (2003) Biol. Chem. 384, 551–566 - PubMed
-
- Müller S. (2004) Mol. Microbiol. 53, 1291–1305 - PubMed
-
- Schirmer R. H., Coulibaly B., Stich A., Scheiwein M., Merkle H., Eubel J., Becker K., Becher H., Müller O., Zich T., Schiek W., Kouyaté B. (2003) Redox Rep. 8, 272–275 - PubMed
-
- Färber P. M., Arscott L. D., Williams C. H., Jr., Becker K., Schirmer R. H. (1998) FEBS Lett. 422, 311–314 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

