Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis
- PMID: 20573978
- PMCID: PMC2921119
- DOI: 10.1091/mbc.E10-05-0421
Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis
Abstract
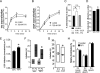
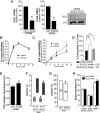
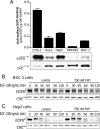
Clathrin-mediated endocytosis (CME) is the main route of internalization of receptor-ligand complexes. Relatively little is known about the role of specific lipids in CME, in particular that of phosphatidic acid (PA). We examined the effect of altering cellular PA levels on CME by manipulating the activities and/or levels of either phospholipase D (PLD1 and PLD2) or diacylglycerol kinase (DGK), two enzyme classes involved in PA production. DGK inhibition resulted in a dramatic reduction of cellular PA, measured directly using an enzyme-coupled reaction, which resulted in a decreased rate of EGFR internalization measured biochemically. This corresponded to a decreased rate of clathrin-coated pit (CCP) initiation and increased lifetimes of productive CCPs, as determined by quantitative live-cell total internal reflection fluorescence microscopy. Unexpectedly, PLD inhibition caused an increase in cellular PA, suggesting that PLD activity negatively regulates PA synthesis by other more productive pathways. Consistent with opposite effects on cellular PA levels, PLD inhibition had opposite effects on EGFR internalization and CCP dynamics, compared with DGK inhibition. Importantly, the constitutive internalization of transferrin receptors was unaffected by either treatment. These findings demonstrate that PA plays a regulatory rather than obligatory role in CME and differentially regulates ligand-stimulated CME of EGFR.
Figures


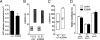
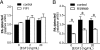
References
-
- Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. - PubMed
-
- Chomczynski P., Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechiques. 1995;19:942–945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

