Regulation of circulating progenitor cells in left ventricular dysfunction
- PMID: 20573992
- PMCID: PMC3096994
- DOI: 10.1161/CIRCHEARTFAILURE.109.879437
Regulation of circulating progenitor cells in left ventricular dysfunction
Abstract
Background: Reductions in numbers of circulating progenitor cells (CD34+ cell subsets) have been demonstrated in patients at risk for, or in the presence of, cardiovascular disease. The mediators of these reductions remain undefined. To determine whether neurohumoral factors might regulate circulating CD34+ cell subsets in vivo, we studied complementary canine models of left ventricular (LV) dysfunction.
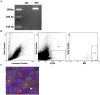
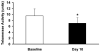
Methods and results: A pacing model of severe LV dysfunction and a hypertensive renal wrap model in which dogs were randomized to receive deoxycorticosterone acetate (DOCA) were studied. Circulating CD34+ cell subsets including hematopoietic precursor cells (HPCs: CD34+/CD45(dim)/VEGFR2-) and endothelial progenitor cells (EPCs: CD34+/CD45-/VEGFR2+) were quantified. Additionally, the effect of mineralocorticoid excess on circulating progenitor cells in normal dogs was studied. The majority of circulating CD34+ cells expressed CD45dimly and did not express VEGFR2, consistent with an HPC phenotype. HPCs were decreased in response to pacing, and this decrease correlated with plasma aldosterone levels (Spearman rank correlation=-0.67, P=0.03). In the hypertensive renal wrap model, administration of DOCA resulted in decreased HPCs. No changes were seen in EPCs in either model. Normal dogs treated with DOCA exhibited a decrease in HPCs in peripheral blood but not bone marrow associated with decreased telomerase activity.
Conclusions: This is the first study to demonstrate that mineralocorticoid excess, either endogenous or exogenous, results in reduction in HPCs. These data suggest that mineralocorticoids may induce accelerated senescence of progenitor cells, leading to their reduced survival and decline in numbers.
Figures






Similar articles
-
Levels of circulating CD45(dim)CD34(+)VEGFR2(+) progenitor cells correlate with outcome in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors.Br J Cancer. 2011 Mar 29;104(7):1144-50. doi: 10.1038/bjc.2011.72. Epub 2011 Mar 8. Br J Cancer. 2011. PMID: 21386843 Free PMC article.
-
High levels of circulating VEGFR2+ Bone marrow-derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies.Clin Cancer Res. 2009 Jul 15;15(14):4561-71. doi: 10.1158/1078-0432.CCR-08-2363. Clin Cancer Res. 2009. PMID: 19605404
-
Circulating CD34+, CD133+, and vascular endothelial growth factor receptor 2-positive endothelial progenitor cells in myelofibrosis with myeloid metaplasia.J Clin Oncol. 2005 Aug 20;23(24):5688-95. doi: 10.1200/JCO.2005.09.021. J Clin Oncol. 2005. PMID: 16110028
-
Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations.Int J Cardiol. 2013 Sep 1;167(5):1688-95. doi: 10.1016/j.ijcard.2012.10.047. Epub 2012 Nov 19. Int J Cardiol. 2013. PMID: 23171577 Review.
-
Update on the pathogenesis of Scleroderma: focus on circulating progenitor cells.F1000Res. 2016 Apr 22;5:F1000 Faculty Rev-723. doi: 10.12688/f1000research.7986.1. eCollection 2016. F1000Res. 2016. PMID: 27158466 Free PMC article. Review.
Cited by
-
Feasibility of frailty screening among patients with advanced heart failure.BMJ Open Qual. 2023 Oct;12(4):e002430. doi: 10.1136/bmjoq-2023-002430. BMJ Open Qual. 2023. PMID: 37857523 Free PMC article.
-
Tachycardia pacing induces myocardial neovascularization and mobilizes circulating endothelial progenitor cells partly via SDF-1 pathway in canines.Heart Vessels. 2016 Feb;31(2):230-40. doi: 10.1007/s00380-014-0613-5. Epub 2014 Dec 10. Heart Vessels. 2016. PMID: 25491934
-
Differential phenotype and behavior in culture of CD34 positive cells from peripheral blood and adipose tissue.Heliyon. 2021 Aug 12;7(8):e07779. doi: 10.1016/j.heliyon.2021.e07779. eCollection 2021 Aug. Heliyon. 2021. PMID: 34458617 Free PMC article. Review.
-
Isolation and Culture of Primary Endothelial Cells from Canine Arteries and Veins.J Vis Exp. 2016 Nov 18;(117):54786. doi: 10.3791/54786. J Vis Exp. 2016. PMID: 27911414 Free PMC article.
References
-
- Wojakowski W, Kucia M, Kazmierski M, Ratajczak MZ, Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27–33. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. - PubMed
-
- Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

