Treatment of geographic atrophy by the topical administration of OT-551: results of a phase II clinical trial
- PMID: 20574018
- PMCID: PMC3055748
- DOI: 10.1167/iovs.10-5637
Treatment of geographic atrophy by the topical administration of OT-551: results of a phase II clinical trial
Abstract
Purpose: To investigate the safety and preliminary efficacy of OT-551, a disubstituted hydroxylamine with antioxidant properties, for the treatment of geographic atrophy (GA), the advanced atrophic form of age-related macular degeneration (AMD).
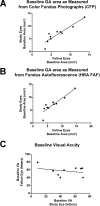
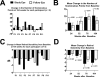
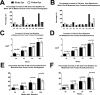
Methods: The study was a single-center, open-label phase II trial, enrolling 10 participants with bilateral GA. Topical 0.45% OT-551 was administered in one randomly assigned eye three times daily for 2 years. Safety measures were assessed by complete ophthalmic examination, fundus photography, and review of symptoms. The primary efficacy outcome measure was the change in best corrected visual acuity at 24 months. Secondary efficacy measures included changes in area of GA, contrast sensitivity, microperimetry measurements, and total drusen area from baseline.
Results: Study drug was well tolerated and was associated with few adverse events. The mean change in BCVA at 2 years was +0.2 ± 13.3 letters in the study eyes and -11.3 ± 7.6 letters in fellow eyes (P = 0.0259). However, no statistically significant differences were found between the study and fellow eyes for all other secondary outcome measures.
Conclusions: OT-551 was well tolerated by study participants and was not associated with any serious adverse effects. Efficacy measurements in this small study indicate a possible effect in maintaining visual acuity. However, the absence of significant effects on other outcomes measures in this study suggests that OT-551, in the current concentration and mode of delivery, may have limited or no benefit as a treatment for GA (ClinicalTrials.gov number, NCT00306488).
Figures



References
-
- Hirvela H, Luukinen H, Laara E, et al. Risk factors of age-related maculopathy in a population 70 years of age or older. Ophthalmology. 1996;103(6):871–877 - PubMed
-
- Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262 - PubMed
-
- Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697–704 - PubMed
-
- Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106(9):1768–1779 - PubMed
-
- Fleckenstein M, Charbel Issa P, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137–4144 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

